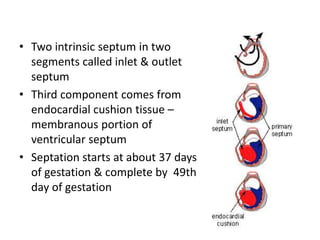
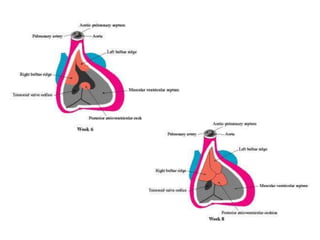
Ventricular septal defects (VSDs) are one of the most common types of congenital heart defects. They occur when there is an abnormal opening in the dividing wall between the lower chambers of the heart (the ventricular septum). VSDs can vary in size and location. Small, restrictive VSDs may close on their own, while larger defects can cause increased blood flow to the lungs which can lead to pulmonary hypertension if left untreated. VSDs are typically diagnosed and monitored using echocardiography. Treatment options range from observation for small defects to surgical closure for larger VSDs.