The document summarizes key concepts about transition elements including:
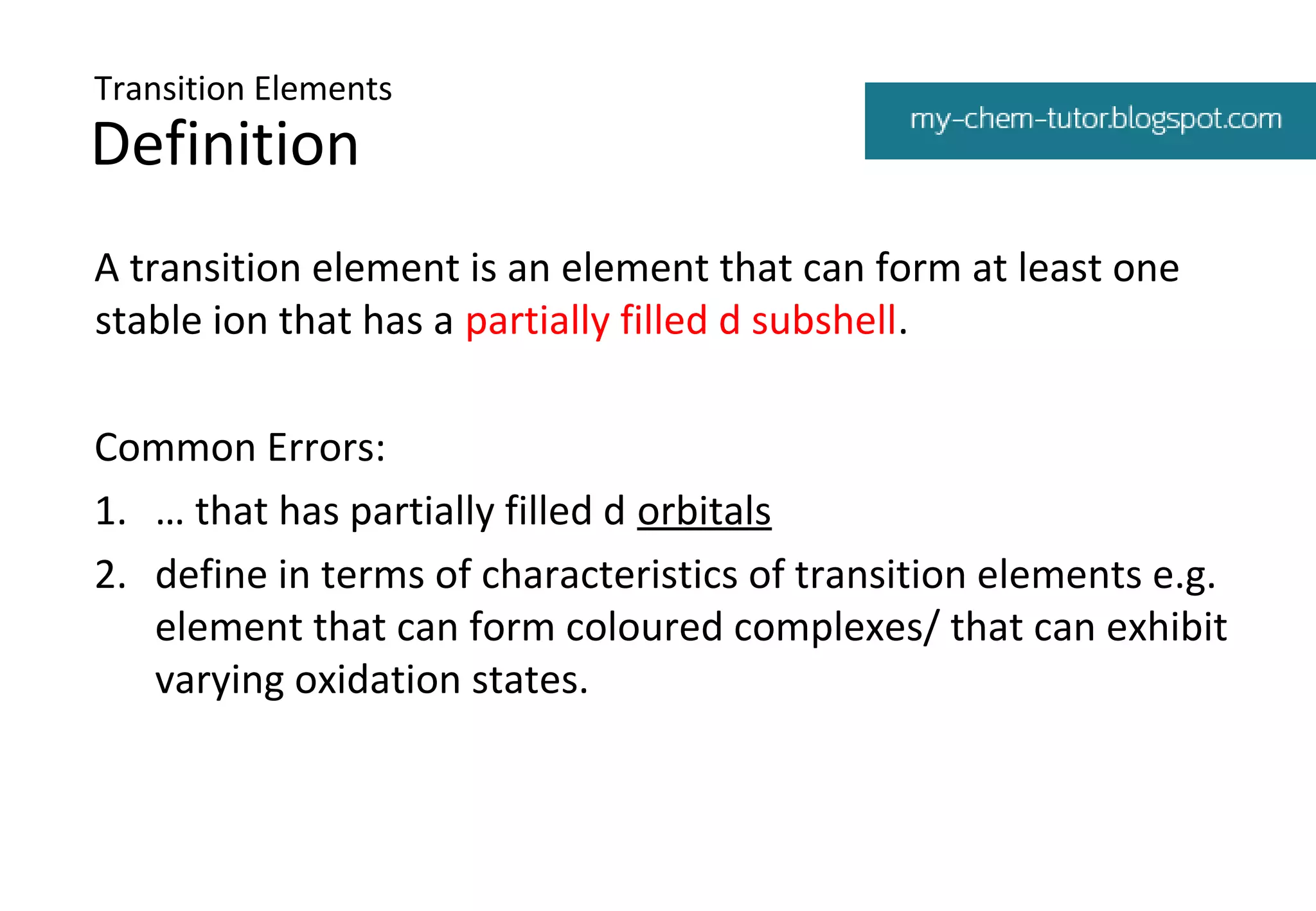
1. Transition elements are defined as elements that can form at least one stable ion with a partially filled d subshell. Common errors in defining transition elements are also discussed.
2. The electronic configurations of transition elements and their ions are covered, noting exceptions like Cr and Cu.
3. Physical properties of transition elements discussed include atomic/ionic radii, ionization energy, melting points and how they vary within the period and group.







![Q: For TM complexes e.g. [Cu(NH3)4(H2O)2]2+, how can the 3d
and 4s accommodate all the lone pairs from the ligands?
A: TM can also use the energetically accessible 4p orbitals for
bonding.
i.e. 3d, 4s and 4p orbitals are available for bonding
9 orbitals that can accommodate up to 18 e–](https://image.slidesharecdn.com/9-140111205757-phpapp01/75/Transition-Elements-10-2048.jpg)





![Q: For aq. metal ions, when do we write out the aqua ligands
and when do we not?
A: It is actually acceptable both ways i.e.
Fe2+ (aq) ≡ [Fe(H2O)6]2+
Cu2+ (aq) ≡ [Cu(H2O)6]2+](https://image.slidesharecdn.com/9-140111205757-phpapp01/75/Transition-Elements-16-2048.jpg)
![The aqua ligands are only shown when they are directly involved
in the reaction to emphasise their presence e.g.
Hydrolysis of water
[Fe(H2O)6]3+ + H2O [Fe(H2O)5(OH)]2+ + H3O+
Ligand exchange
[Fe(H2O)6]3+ + 6NH3 [Fe(NH3)6]3+ + 6H2O](https://image.slidesharecdn.com/9-140111205757-phpapp01/75/Transition-Elements-17-2048.jpg)

