Embed presentation
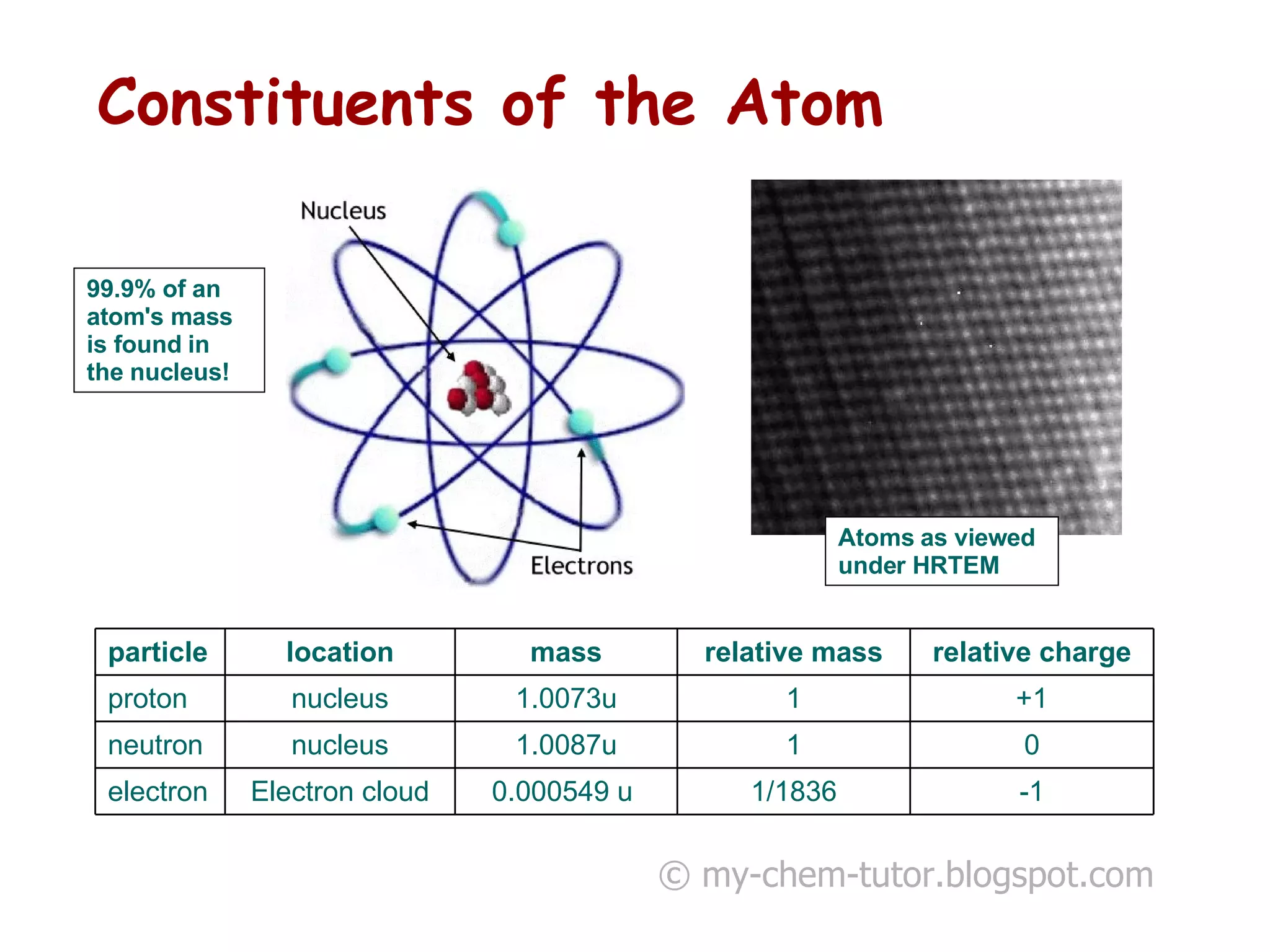
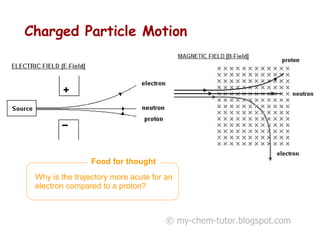
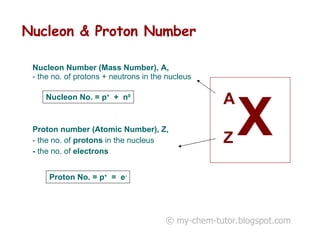
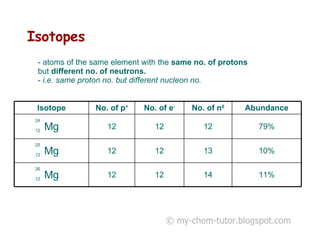
The document discusses the basic constituents of atoms: electrons, protons, and neutrons. It notes that electrons have a negative charge and small mass, protons have a positive charge and greater mass, and neutrons have no charge and a mass similar to protons. Almost all an atom's mass is contained in the nucleus, which is made up of protons and neutrons. The document also defines key atomic concepts like nucleon number, proton number, and isotopes.



