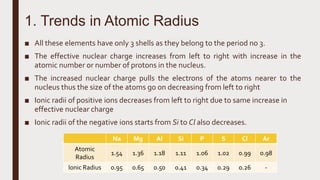
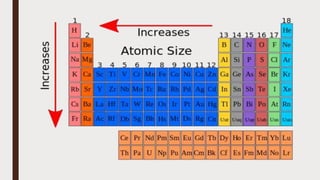
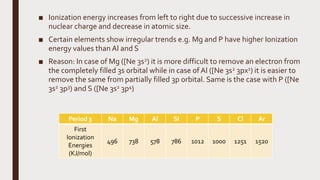
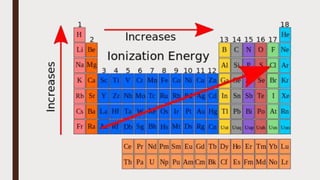
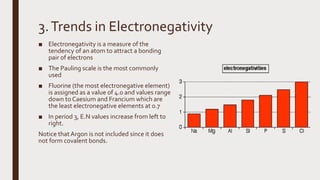
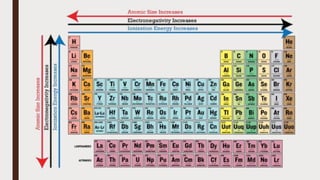
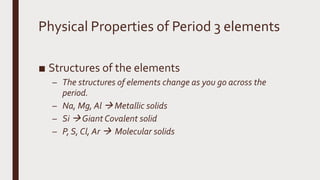
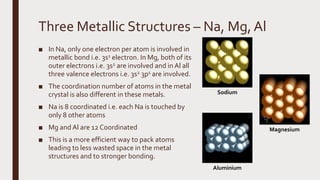
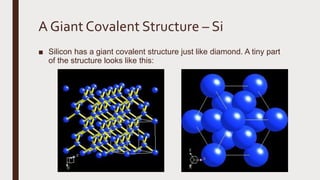
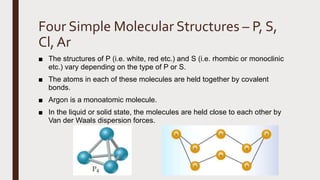
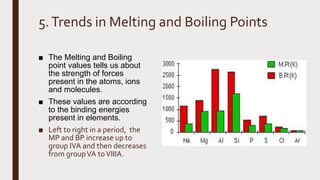
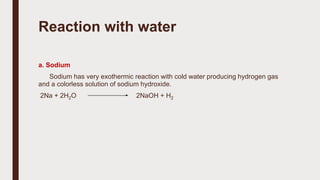
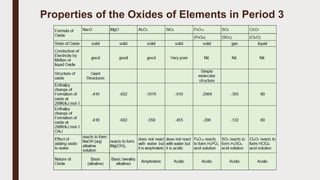
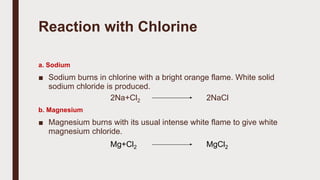
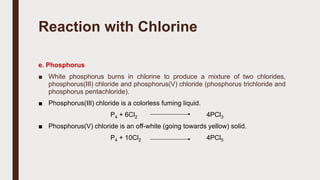
The document discusses the atomic properties of period 3 elements, including their electronic structure, trends in atomic radius, first ionization energy, electronegativity, physical properties, electrical conductivity, and chemical reactions with water and oxygen. It covers how these elements behave in terms of size, energy requirements for ionization, and their chemical reactions under various conditions. Additionally, it details the types of structures formed by these elements and their reactivity with other substances, such as chlorine.

![Sodium Na
1s2 2s2 2p6 3s1
Or [Ne] 3s1](https://image.slidesharecdn.com/spblockelements-171204161014/85/S-p-block-elements-4-320.jpg)
![Magnesium
Mg
[Ne] 3s2](https://image.slidesharecdn.com/spblockelements-171204161014/85/S-p-block-elements-5-320.jpg)
![Aluminium Al
[Ne] 3s2 3px1](https://image.slidesharecdn.com/spblockelements-171204161014/85/S-p-block-elements-6-320.jpg)
![Silicon Si
[Ne] 3s2 3px1 3py1](https://image.slidesharecdn.com/spblockelements-171204161014/85/S-p-block-elements-7-320.jpg)
![Phosphorous
P
[Ne] 3s2 3px1 3py1 3pz1](https://image.slidesharecdn.com/spblockelements-171204161014/85/S-p-block-elements-8-320.jpg)
![Sulphur S
[Ne] 3s2 3px2 3py1 3pz1](https://image.slidesharecdn.com/spblockelements-171204161014/85/S-p-block-elements-9-320.jpg)
![Chlorine Cl
[Ne] 3s2 3px2 3py2 3pz1](https://image.slidesharecdn.com/spblockelements-171204161014/85/S-p-block-elements-10-320.jpg)
![Argon Ar
[Ne] 3s2 3px2 3py2 3pz2](https://image.slidesharecdn.com/spblockelements-171204161014/85/S-p-block-elements-11-320.jpg)