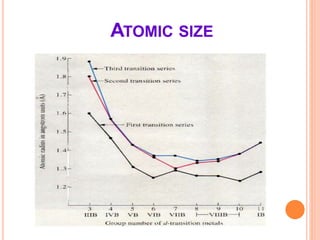
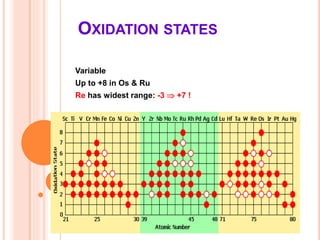
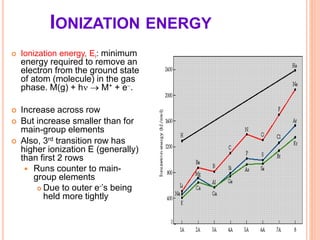
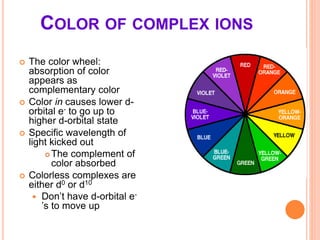
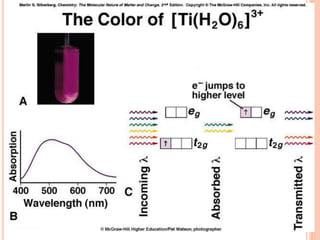
This document discusses transition series elements and their properties. It describes how transition elements have electrons that enter the (n-1)d orbitals, giving them variable oxidation states up to +8. Their atomic radii decrease across periods but increase down groups. Transition metals can conduct heat and electricity well and can be alloyed to improve strength. Some have magnetic properties depending on unpaired electrons. Their colored complexes are due to electron transitions between d orbitals. Common applications include stainless steel, bronze, and uses of copper and nickel in coins, batteries, and turbines.