This document summarizes the physical and chemical properties of Group VII (halogen) elements. It describes their:
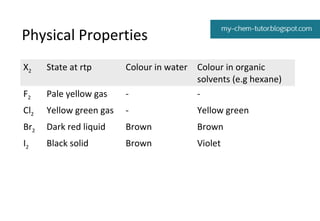
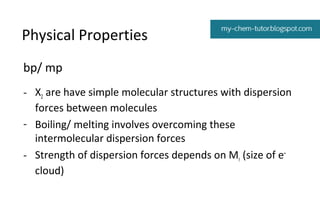
1) Colors and states - the halogens range from pale yellow gas (F2) to black solid (I2).
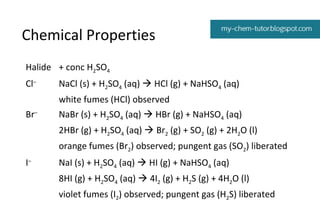
2) Reactivity trends - fluorine is the strongest oxidizing agent while iodine is the weakest. Fluoride is the weakest reducing agent and iodide is the strongest.
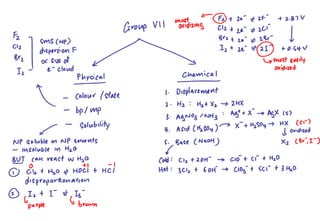
3) Reactions with silver nitrate and ammonia - these tests can be used to identify halide ions based on solubility differences of the precipitates formed.





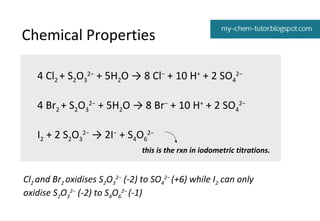


![Chemical Properties
Halide
+ AgNO3 (aq)
+ NH3
Cl– (aq)
white ppt
soluble in aq NH3
Br– (aq)
cream ppt
insoluble in aq NH3; soluble in conc
NH3
I– (aq)
yellow ppt
insoluble in aq NH3 and conc NH3
Ag+ + X– AgX
(s)
AgX (s) ⇌ Ag+ + X–
Ag+ + 2NH3 ⇌ [Ag(NH3)2]+](https://image.slidesharecdn.com/9-140111205732-phpapp01/85/Group-VII-14-320.jpg)