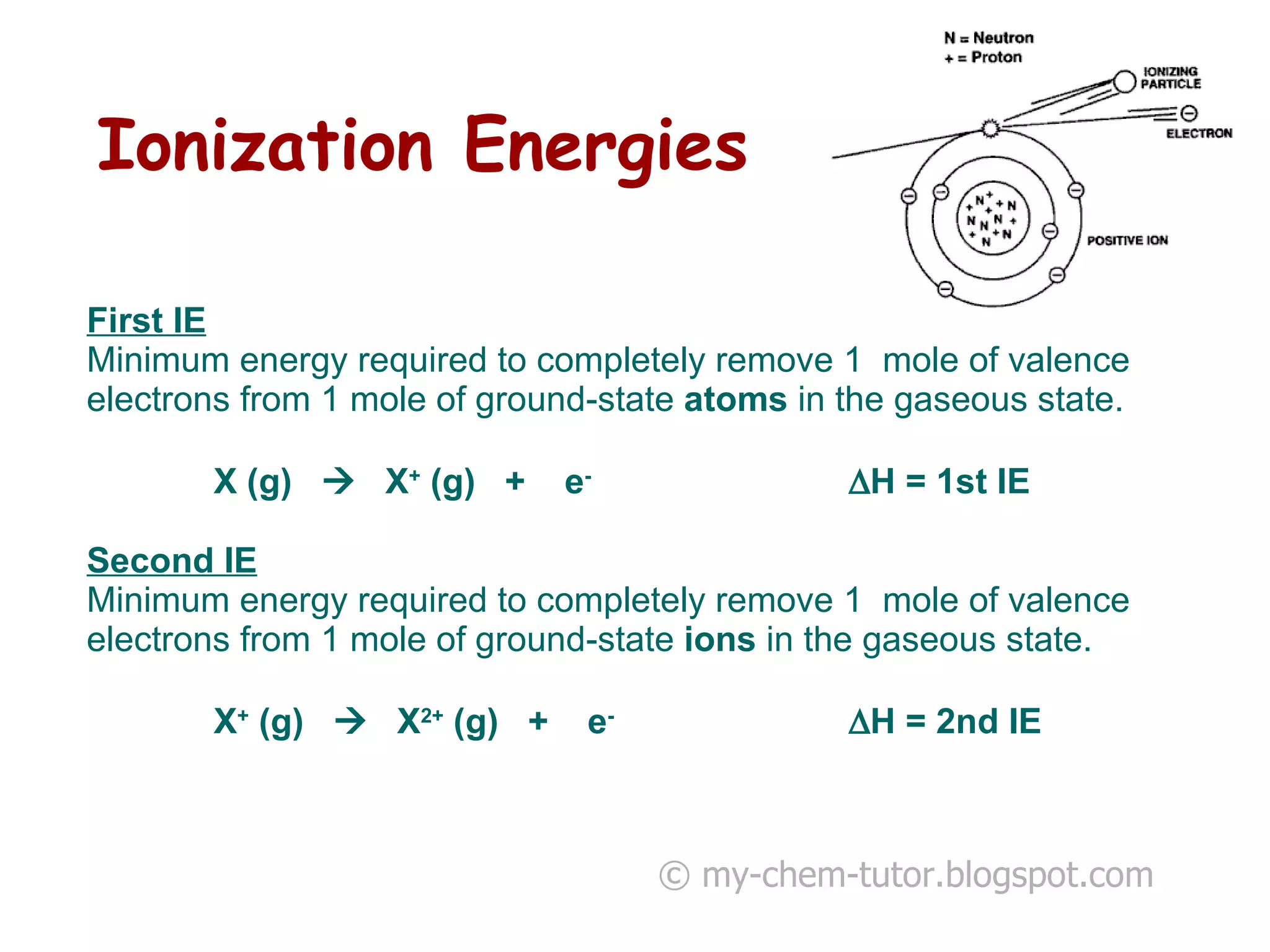
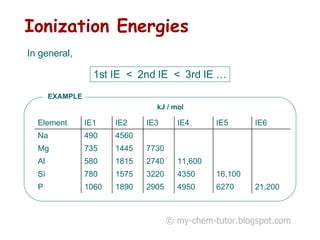
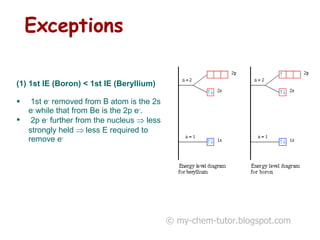
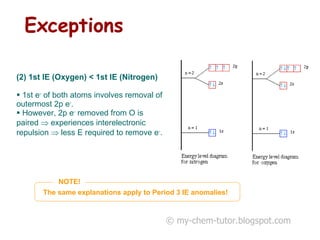
1) The first ionization energy (IE) is the minimum energy required to remove one mole of valence electrons from one mole of ground state atoms. Successive IEs require removing electrons from ionized atoms.
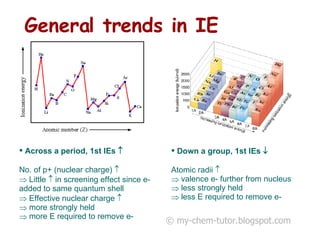
2) General trends in IEs include them decreasing down a group as atomic radius increases, and increasing across a period as nuclear charge increases.
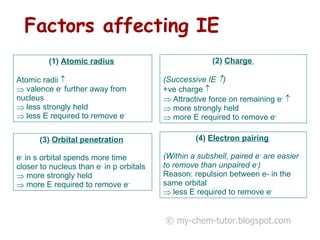
3) Factors like electron pairing, charge, and orbital penetration affect IEs by influencing how strongly electrons are held.