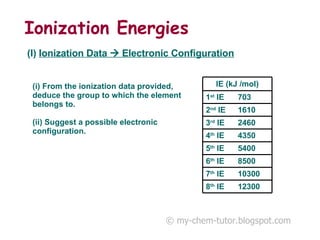
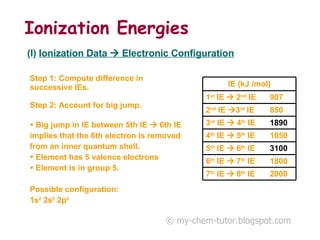
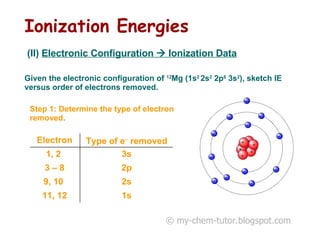
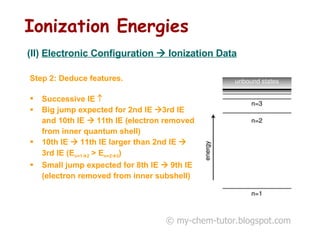
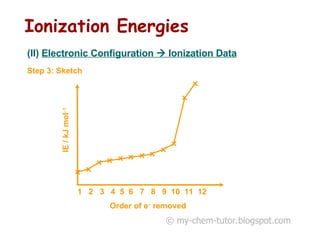
The document discusses interpreting ionization energy (IE) data to determine an element's electronic configuration. It states that a large jump in IE indicates removal of an electron from an inner shell, while a small jump indicates removal from an inner subshell. It provides an example of using IE data to deduce an element is in group 5 with a possible configuration of 1s22s22p5. It also shows how the electronic configuration of magnesium can be used to sketch its IE trend by determining what type of electron is removed at each stage.