Embed presentation
Downloaded 13 times


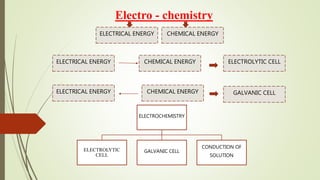
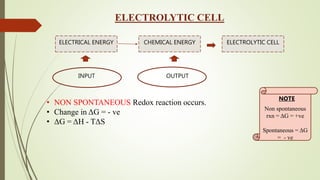

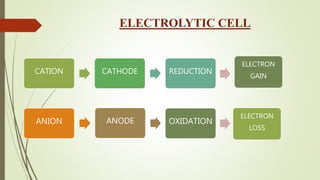

This document discusses electrochemistry and different types of electrochemical cells. It explains that in an electrolytic cell, electrical energy is used to drive a non-spontaneous redox reaction to produce a chemical energy output. The cathode is where reduction occurs through electron gain, and the anode is where oxidation occurs through electron loss. An electrolytic solution contains molecules bound by ionic bonds that allow conduction when an electrical current is applied to the cell.






