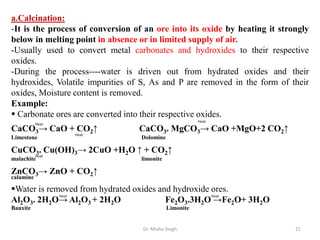
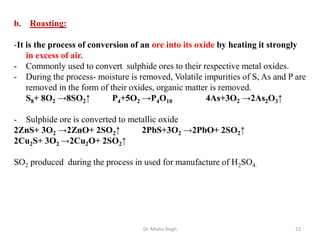
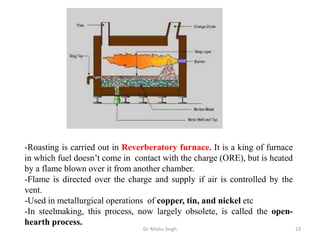
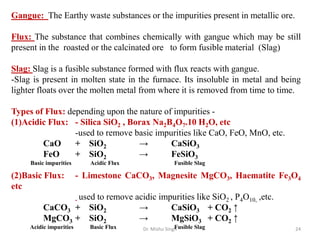
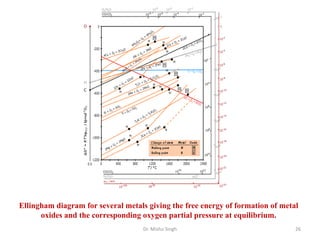
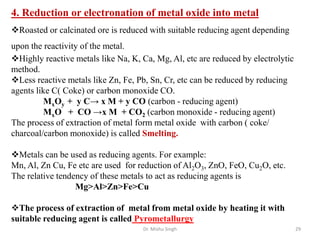
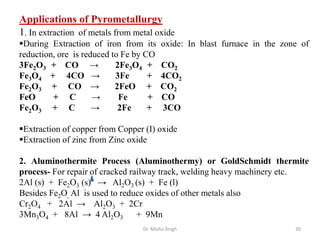
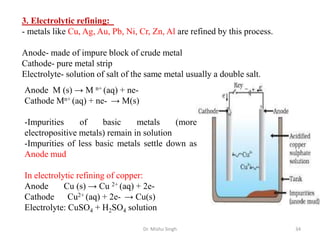
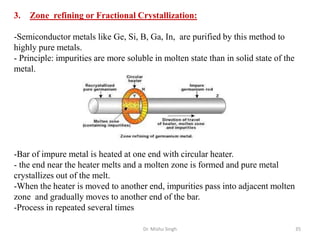
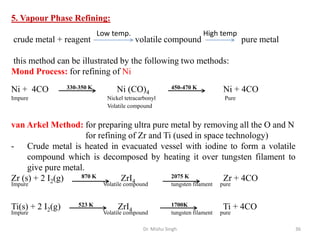
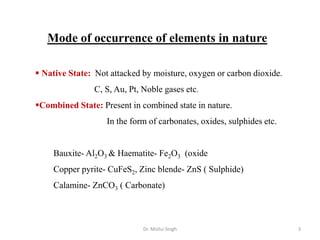
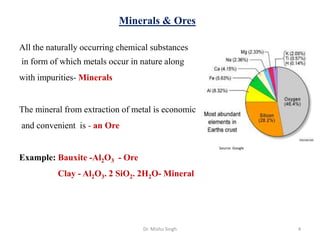
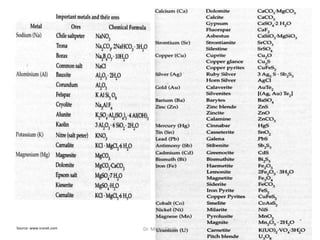
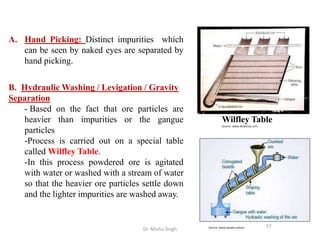
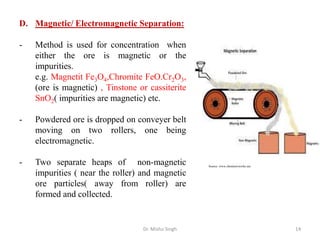
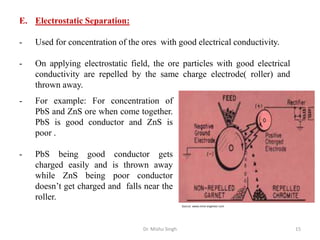
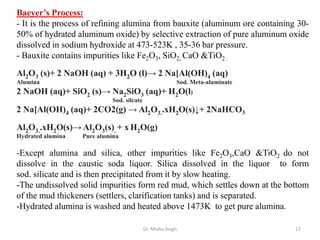
The document discusses various principles and processes involved in the isolation of elements through metallurgy. It describes how elements are found in nature, either in native state or combined state in minerals and ores. It then explains the metallurgical processes of crushing and grinding ores, concentrating the ore through various methods, converting the concentrated ore into metal oxides through calcination or roasting. Finally, it discusses reducing the metal oxides into metals through reduction processes using suitable reducing agents, based on the reactivity and position of metals in the Ellingham diagram.










![Dr. Mishu Singh 18
Cyanide Leaching of Silver and Gold - Mac Arthur Forest Cyanide Process
- Cyanide leaching is the most important process developed for extraction of
precious metals like Au & Ag as it produces pure metal as a final product.
- In the process Argentite or Native Au or Ag is treated with 0.5% NaCN or
KCN solution.
-Metals gets oxidized and forms soluble cyanide complex.
4 M+ 8 CN- + 2H2O+ O2→ 4[M(CN)2
-]+ 4 OH-
(air)
a. For Silver:
(i)When native Silver metal is taken for the process-
4 Ag+ 8 NaCN + 2H2O+ O2→ 4 Na[Ag(CN)2]+ 4 NaOH
(air)
(ii)When Argentite or silver glance (Ag2S) is taken, the initial reaction of
formation of soluble silver complex formed is reversible. Here, the current
of air oxidizes Na2S to Na2SO4
Ag2S+ 4 NaCN 2 Na[Ag(CN)2]+ Na2S
Sod. Dicyanoargentite(I)
Soluble complex
b. For Gold:
4 Au+ 8 KCN + 2H2O+ O2→ 4K[M(CN)2]+ 4 KOH
(air)](https://image.slidesharecdn.com/metallurgy-200420075452/85/Metallurgy-18-320.jpg)