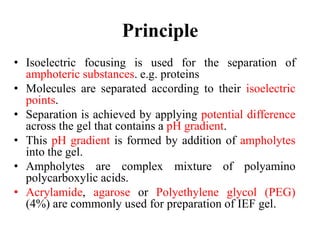
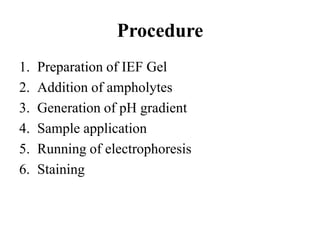
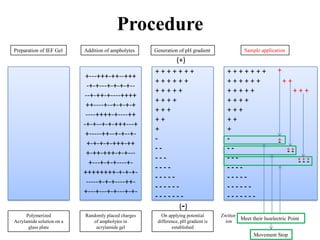
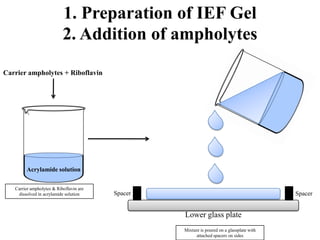
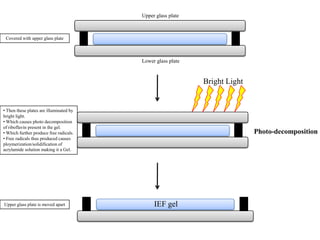
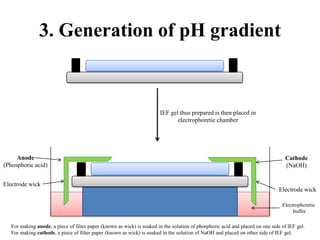
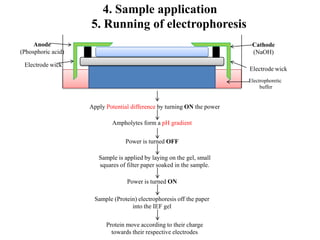
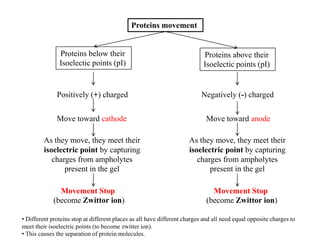
Isoelectric focusing is a technique used to separate proteins based on their isoelectric point. It involves creating an immobilized pH gradient using carrier ampholytes within an acrylamide gel. When an electric current is applied, proteins will migrate within the gel until they reach the point where they carry no net charge and stop, allowing separation based on subtle differences in pI. The key steps are preparation of the IEF gel, addition of ampholytes to generate the pH gradient, running electrophoresis to allow protein migration, and staining to visualize the separated protein bands.