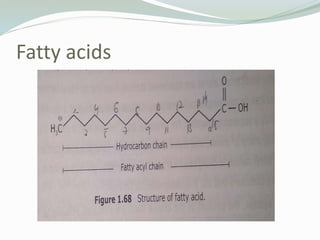
The document provides a comprehensive overview of various types of lipids, including fatty acids, triacylglycerols, phospholipids, glycolipids, and eicosanoids, detailing their structures, functions, and classifications. It explains the differences between saturated and unsaturated fatty acids, the roles lipids play in biological systems, and their significance in membrane formation, energy storage, and signaling. Essential fatty acids and the structure-function relationship of different lipid classes, including their physiological and clinical implications, are also discussed.