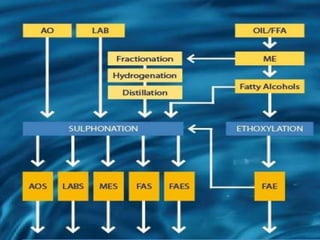
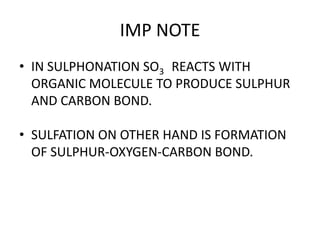
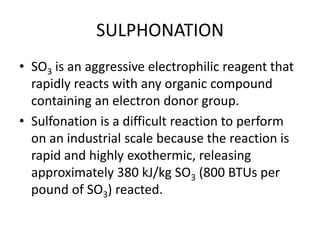
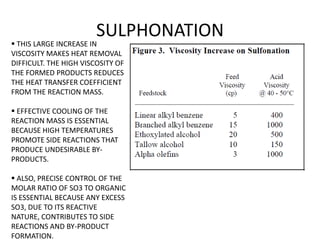
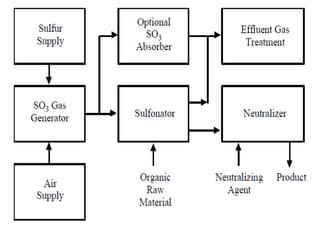
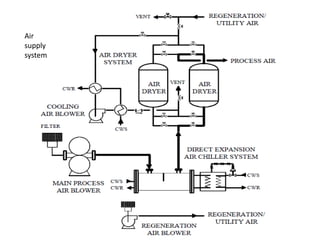
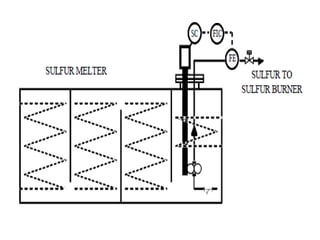
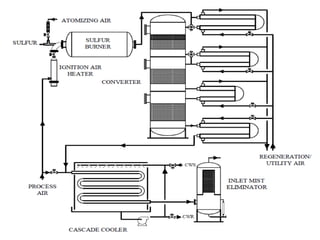
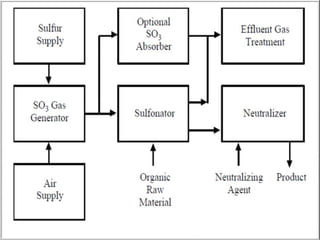
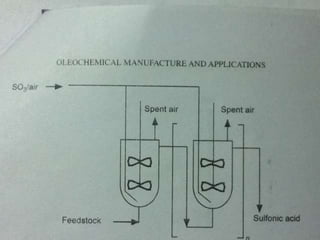
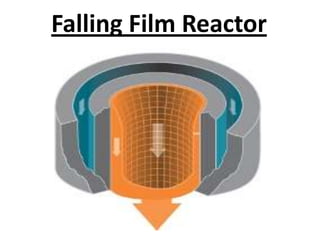
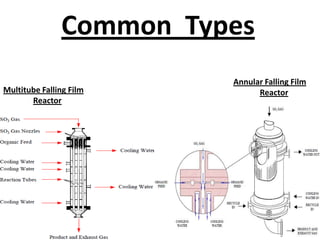
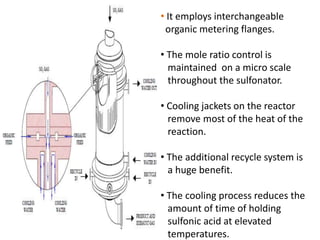
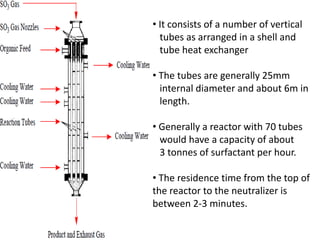
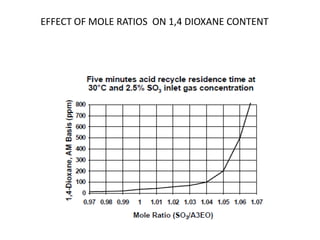
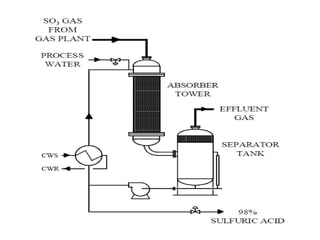
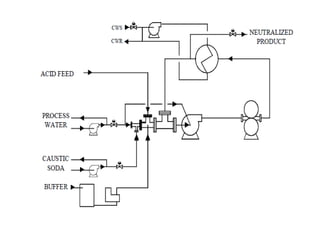
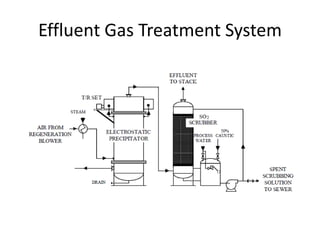
This document discusses sulfonation, which is the reaction of an organic compound with sulfur trioxide to form a sulfonic acid. Sulfonation is an exothermic and rapid reaction that is difficult to control industrially. The document outlines the key components of an industrial sulfonation process, including systems for air supply, sulfur feeding, sulfur burning to generate SO3, a falling film reactor, SO3 absorption, neutralization, and effluent gas treatment. Precise control of temperature, mole ratios, and residence time are required to optimize the process and minimize byproducts.