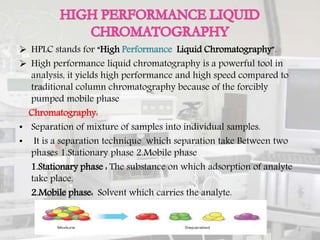
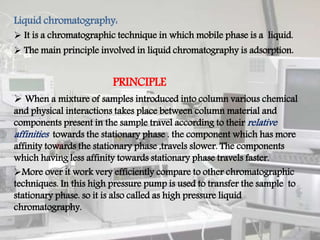
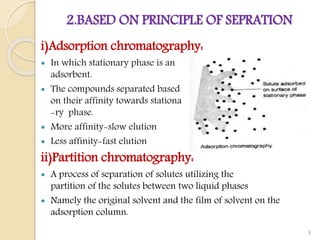
This document provides an overview of high performance liquid chromatography (HPLC). It begins by defining HPLC and explaining that it uses high pressure to pump the mobile phase, yielding faster separation than traditional column chromatography. The document then discusses the basic principles of chromatography and liquid chromatography. It provides details on the types of HPLC based on mode of separation, principle of separation, elution technique, scale of operation, and type of analysis. The key components of an HPLC instrument are described including the solvent reservoir, pump, injector, column, detectors, and data recording system. Various columns, stationary phases, and pumps used in HPLC are also outlined.







![I. Solvent reservoir & Mixing unit & degassing
system
The appropriate solvents [mobile phase] from the reservoirs
are allowed to enter the mixing chamber where a
homogenous mixture is obtained.
Several gases are soluble in organic solvents.
When solvents are pumped under high pressure, gas
bubbles are formed which will interfere with the separation
process, steady base line and the shape of the peak.
Hence degassing of solvent is important. This can be done by
-Vacuum filtration,
-Helium purging,
-Ultrasonication.
14](https://image.slidesharecdn.com/manojfinalppt-171110163528/85/HPLC-BY-MANOJ-KUMAR-M-14-320.jpg)




![IV. Column
The column [stationary phase] separates the sample components of
interest using various physical and chemical parameters.
The columns used for HPLC are generally made up of stainless steel .
so they can withstand up to high pressure [8000psi].
Straight columns of 20-50 cm in length & 1-4mm in diameter are
used .
Particle size should be for porous particles 20-40μm. For porous
micro particles it should be 3-10 μm.
porous plugs of stainless steel are used in ends of column to retain
the packing material.
Stationary phases used in column:
-Alumina
-Silica
-Polystyrene
-Polyvinyl acetate beads 20](https://image.slidesharecdn.com/manojfinalppt-171110163528/85/HPLC-BY-MANOJ-KUMAR-M-20-320.jpg)

![COLUMN EFFICIENCY IN SEPARATION PROCESS
A. Theoretical plates:
Chromatographic column contains a large number of separate
layers, called ‘Theoretical Plates’.
Separate equilibrations of the sample between the stationary and
mobile phase occur in these "plates".
The analyte moves down the column by transfer of equilibrated
mobile phase from one plate to the next.
It is important to remember that the plates do not really exist ; they
are a figment of the imagination that helps us understand the
processes at work in the column.
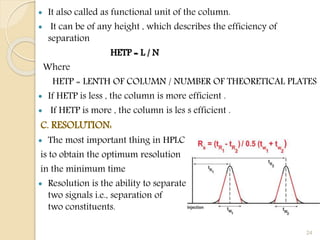
B. HETP [Height Equivalent to a Theoretical plate]:
A theoretical plate is an imaginary unit of a column, where
distribution of solute between , stationary phase and mobile phase,
has attained equilibrium.
23](https://image.slidesharecdn.com/manojfinalppt-171110163528/85/HPLC-BY-MANOJ-KUMAR-M-23-320.jpg)




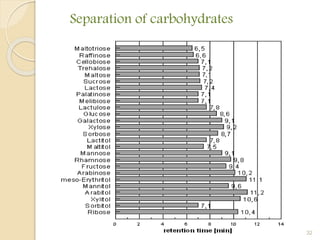
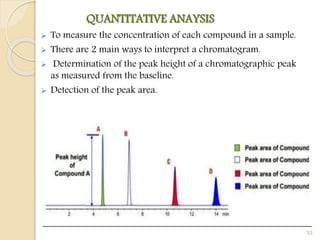
![QUALITATIVE ANALYSIS
The identification of individual compounds in the sample.
The most common parameter for compound identification is its
Retention Time [Is the amount of time a compound spends on
the column after injection]
Depending on the detector used, compound. identification is
also based on the chemical structure, molecular weight or some
other molecular parameter.
34](https://image.slidesharecdn.com/manojfinalppt-171110163528/85/HPLC-BY-MANOJ-KUMAR-M-34-320.jpg)

