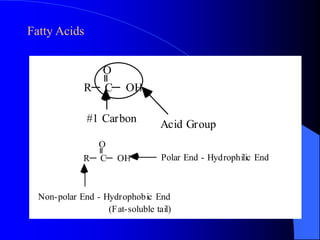
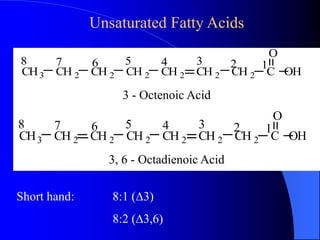
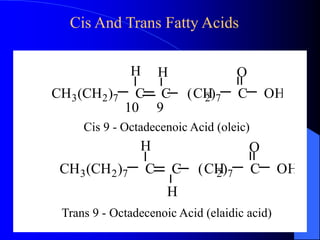
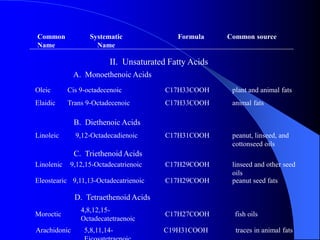
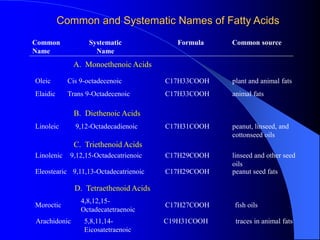
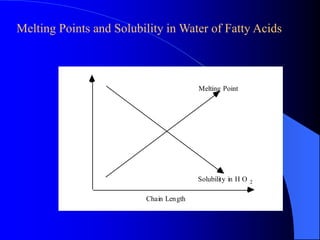
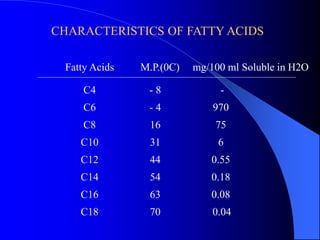
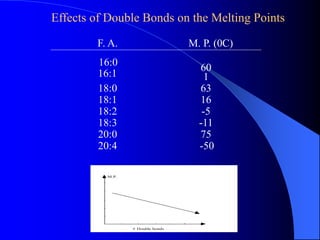
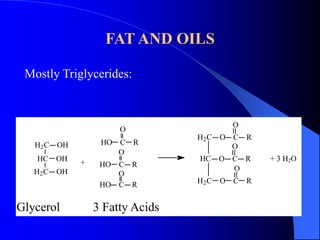
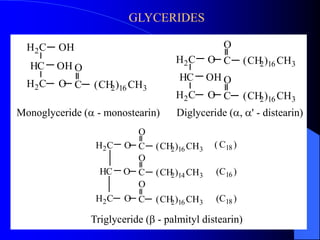
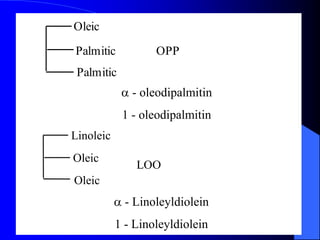
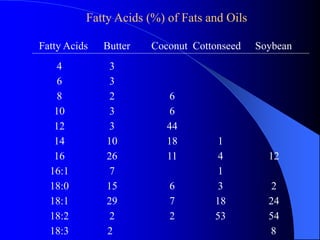
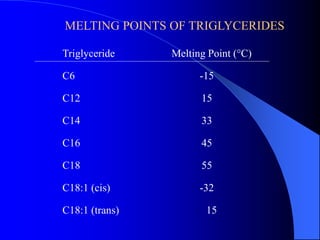
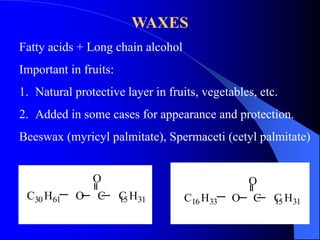
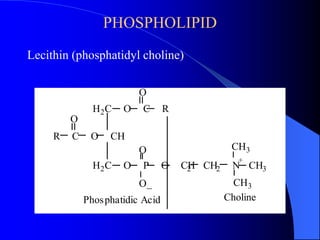
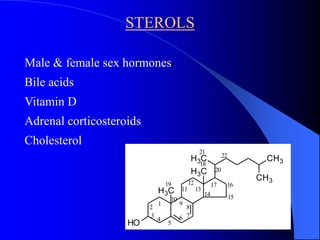
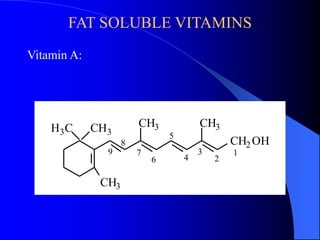
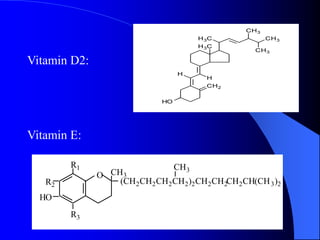
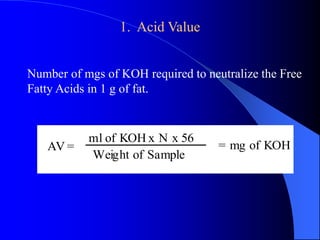
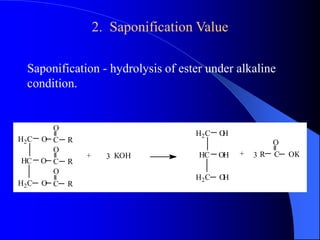
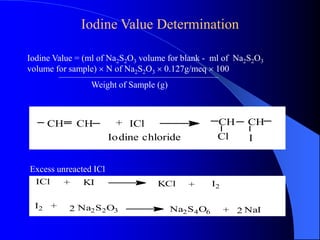
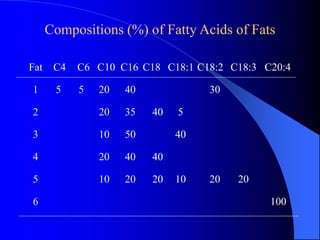
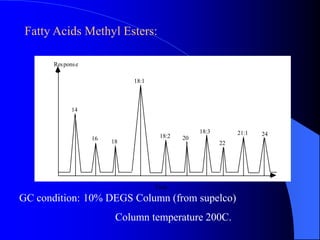
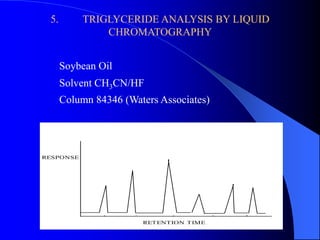
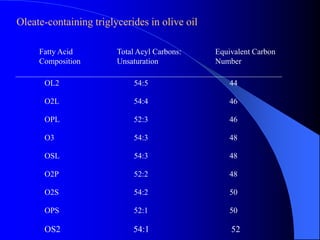
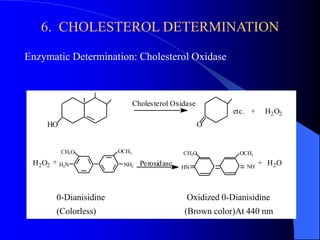
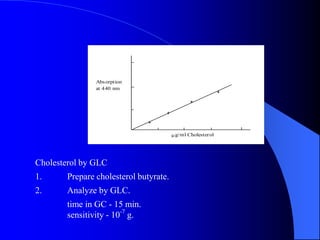
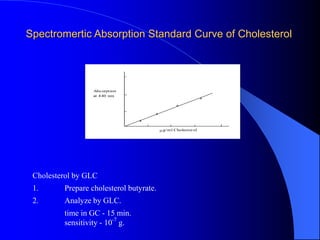
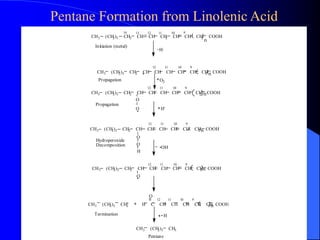
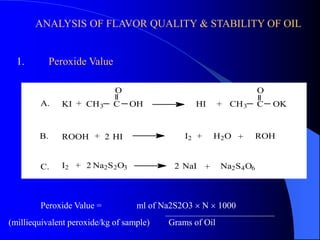
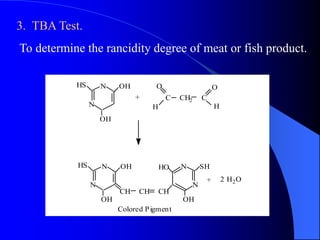
This document summarizes the key characteristics and types of lipids. Lipids are insoluble in water but soluble in non-polar solvents. The main types of lipids discussed include fatty acids, neutral fats and oils, waxes, phospholipids, sterols, and fat-soluble vitamins. Specific lipids like triglycerides, cholesterol, and various fatty acids are examined in detail. Analytical methods for analyzing lipids, such as acid value, saponification value, iodine number, gas chromatography, and enzymatic assays are also outlined.