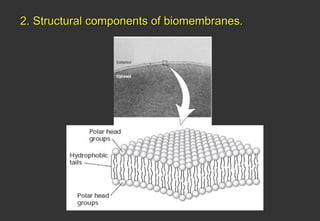
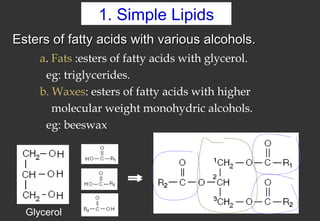
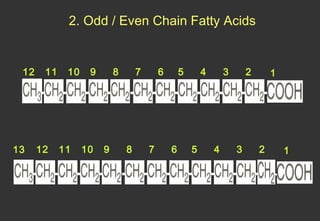
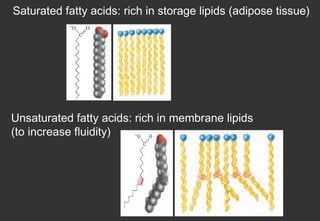
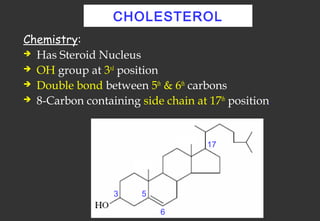
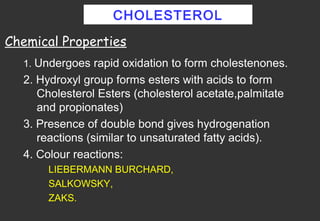
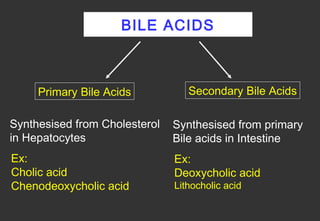
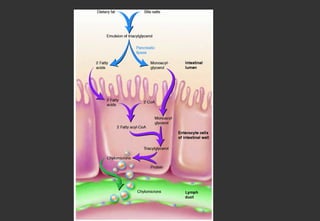
Lipids are a class of compounds that are insoluble in water but soluble in nonpolar solvents, and include fats, waxes, phospholipids, glycolipids, and other complex lipids. Lipids serve important structural and functional roles in the body such as energy storage, components of cell membranes, insulation, and producing hormones and signaling molecules. Lipids are also involved in many diseases when levels become abnormal.