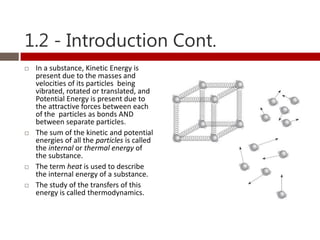
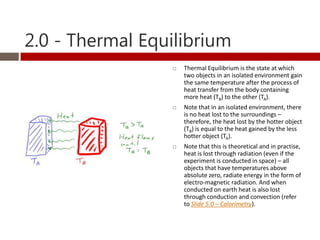
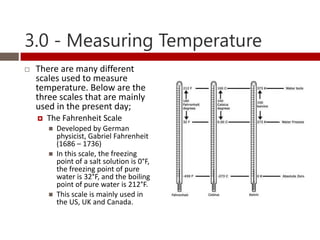
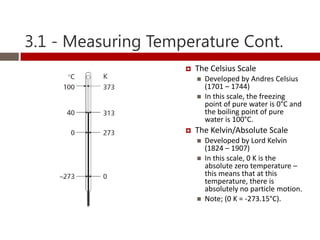
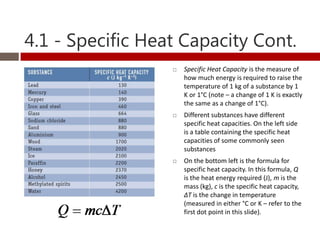
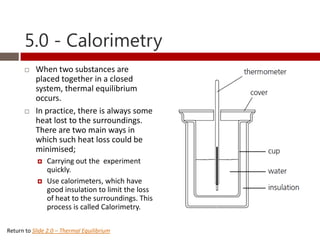
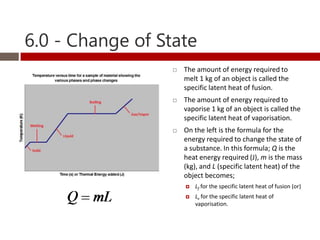
Heat and temperature are different concepts. Heat is a form of energy measured in Joules, while temperature is a measure of the average kinetic energy of particles measured in Kelvin or Celsius. Objects can contain various forms of energy including kinetic energy from motion and potential energy from forces between particles. Thermal equilibrium occurs when objects in contact reach the same temperature after heat transfer. Specific heat capacity is the amount of energy required to change an object's temperature and depends on the material. Phase changes from solid to liquid or liquid to gas require latent heat and occur when particles gain enough energy to overcome attractive forces.