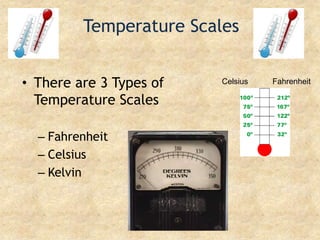
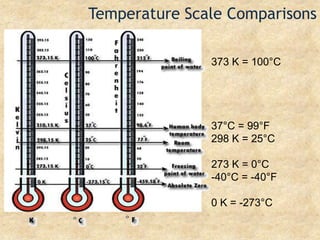
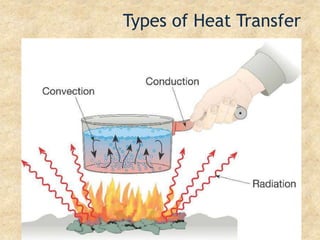
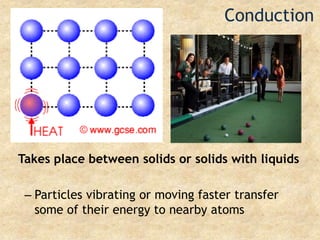
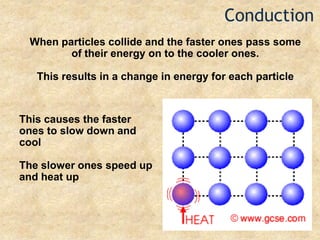
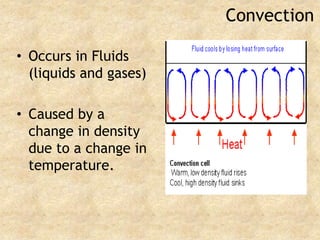
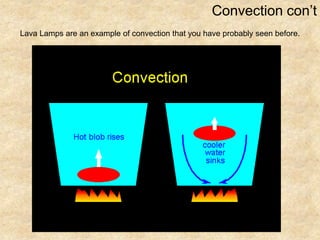
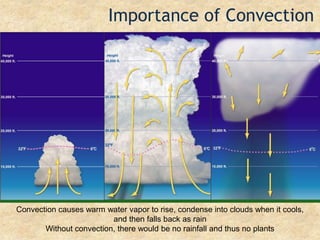
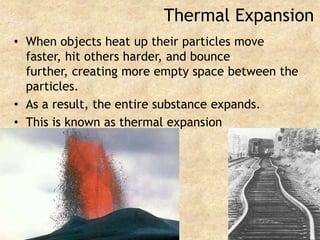
Temperature is a measure of the average kinetic energy of particles, with higher temperatures indicating faster particle motion. There are three main temperature scales: Fahrenheit, Celsius, and Kelvin. Fahrenheit and Celsius are used to measure temperatures experienced in daily life, while Kelvin is used for scientific purposes since it does not have negative values. Heat is transferred between objects through conduction, convection, and radiation. Conduction requires direct contact, convection occurs through fluid movement, and radiation transfers heat via electromagnetic waves.