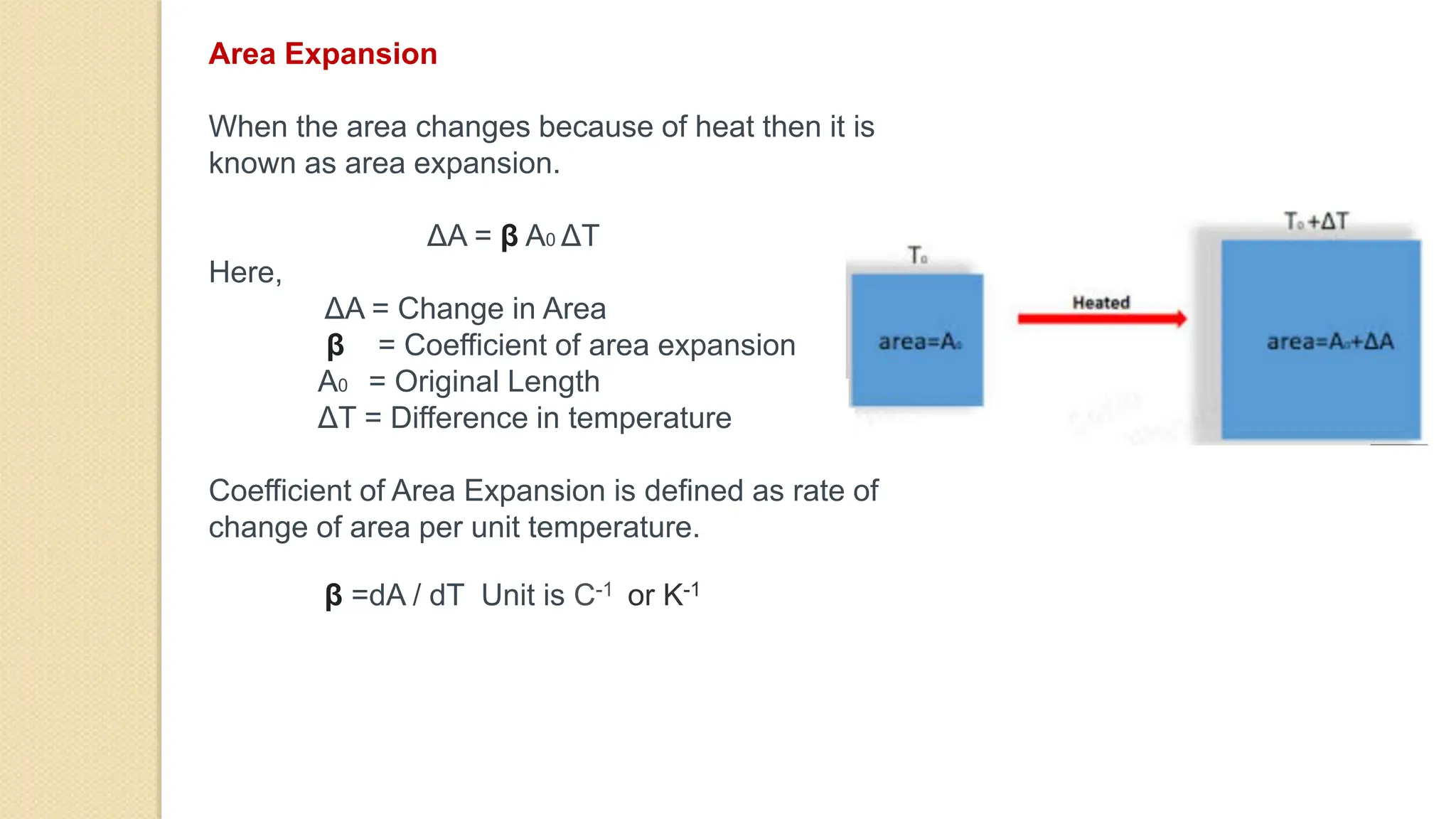
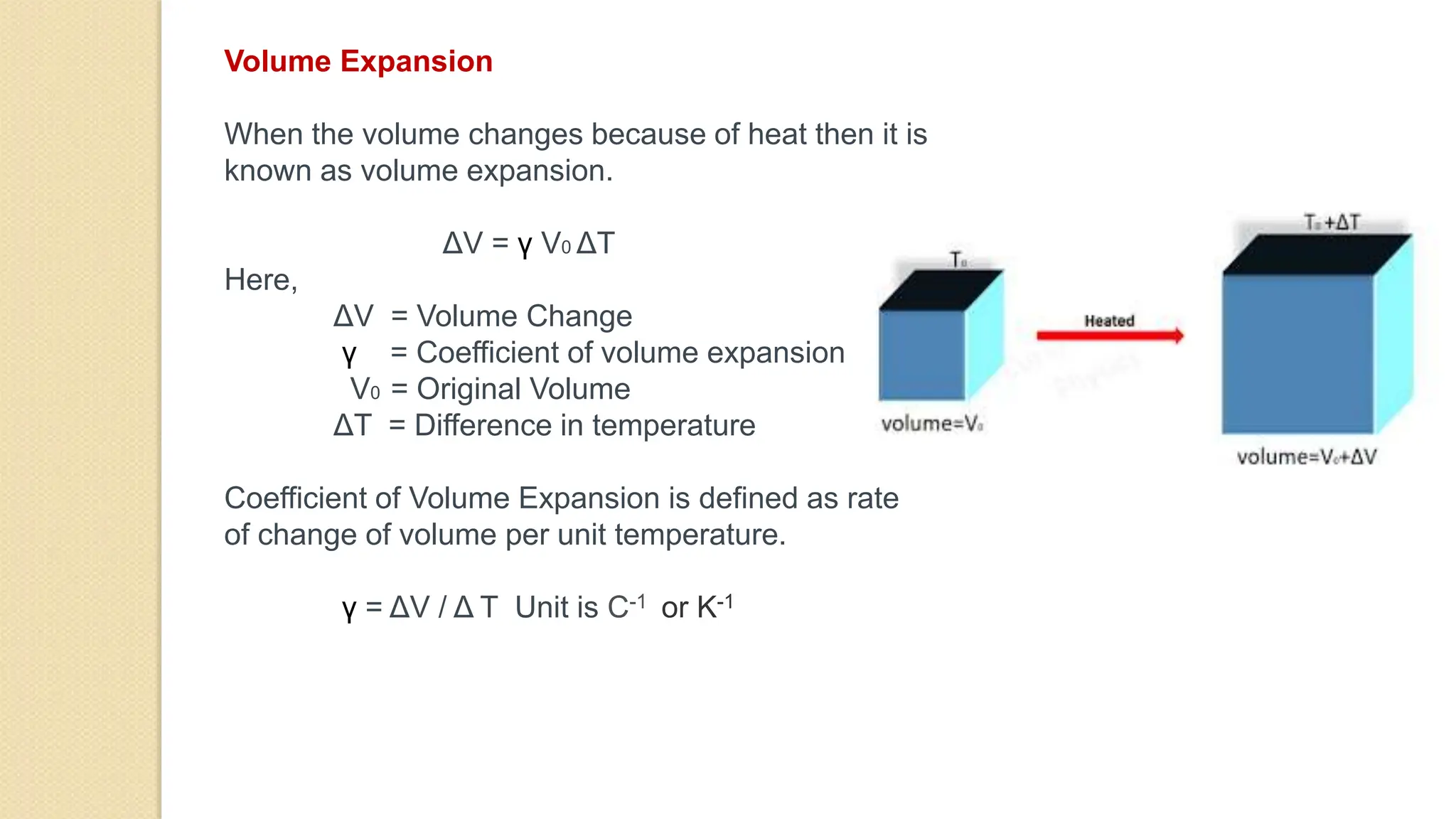
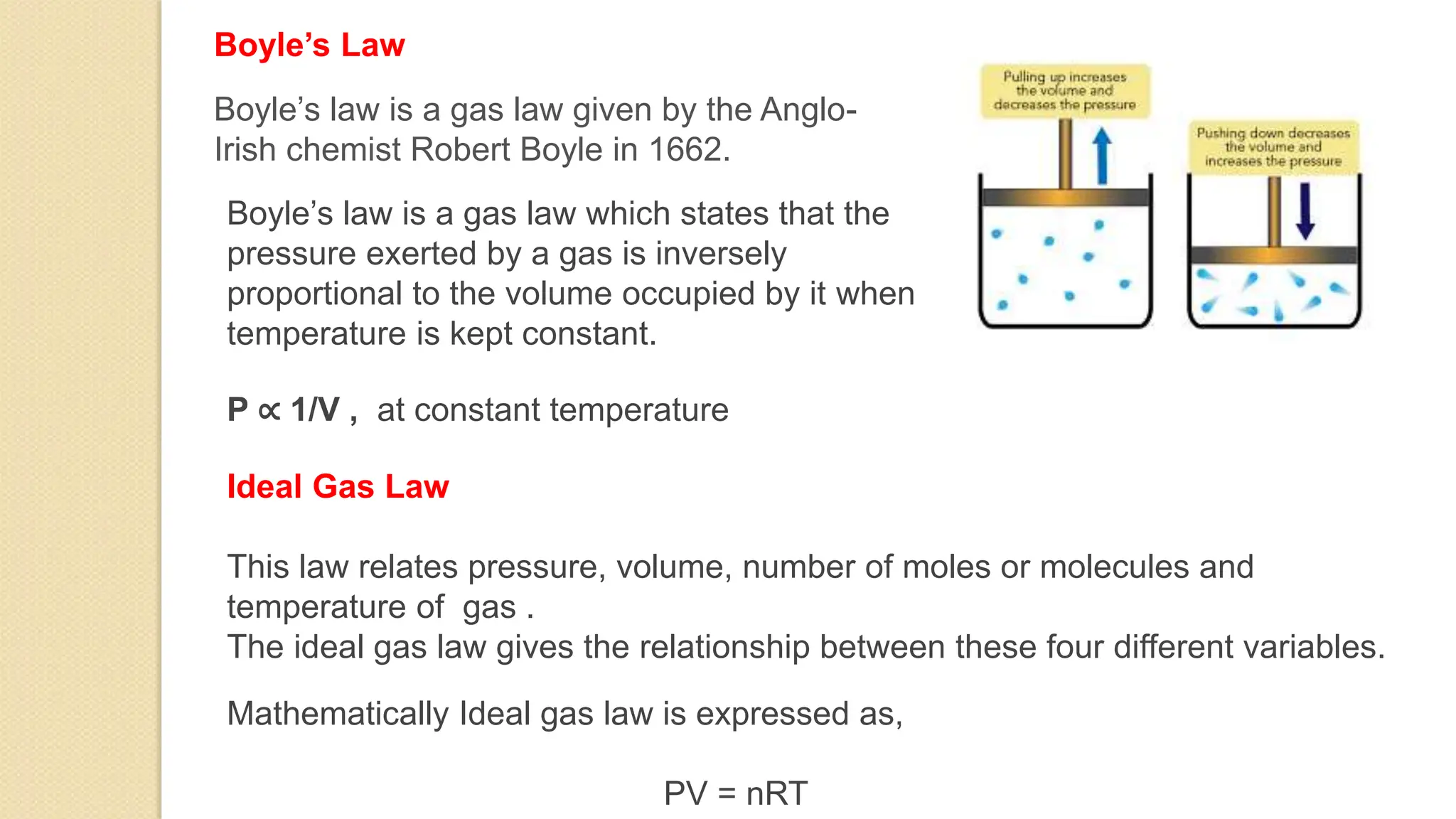
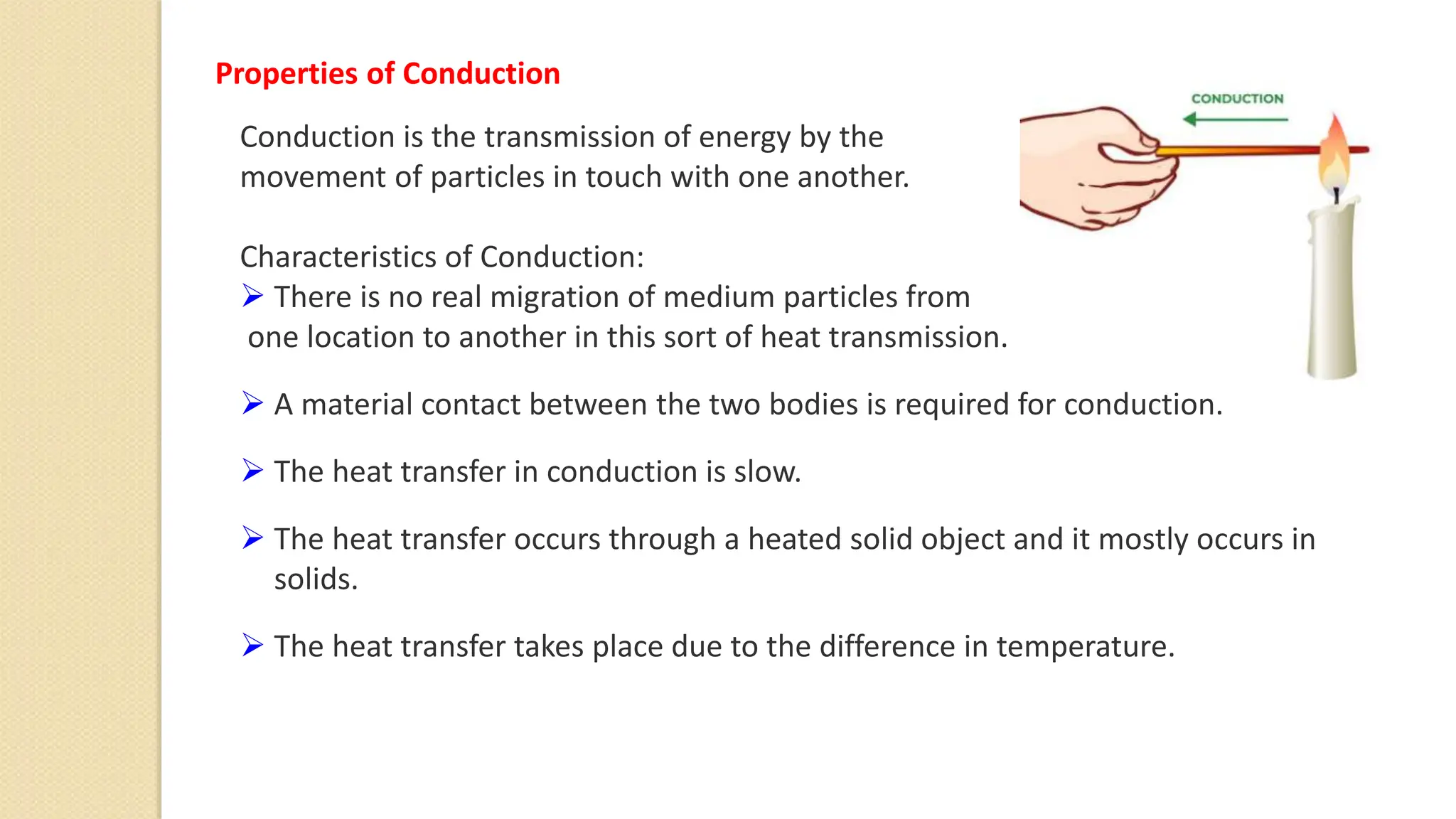
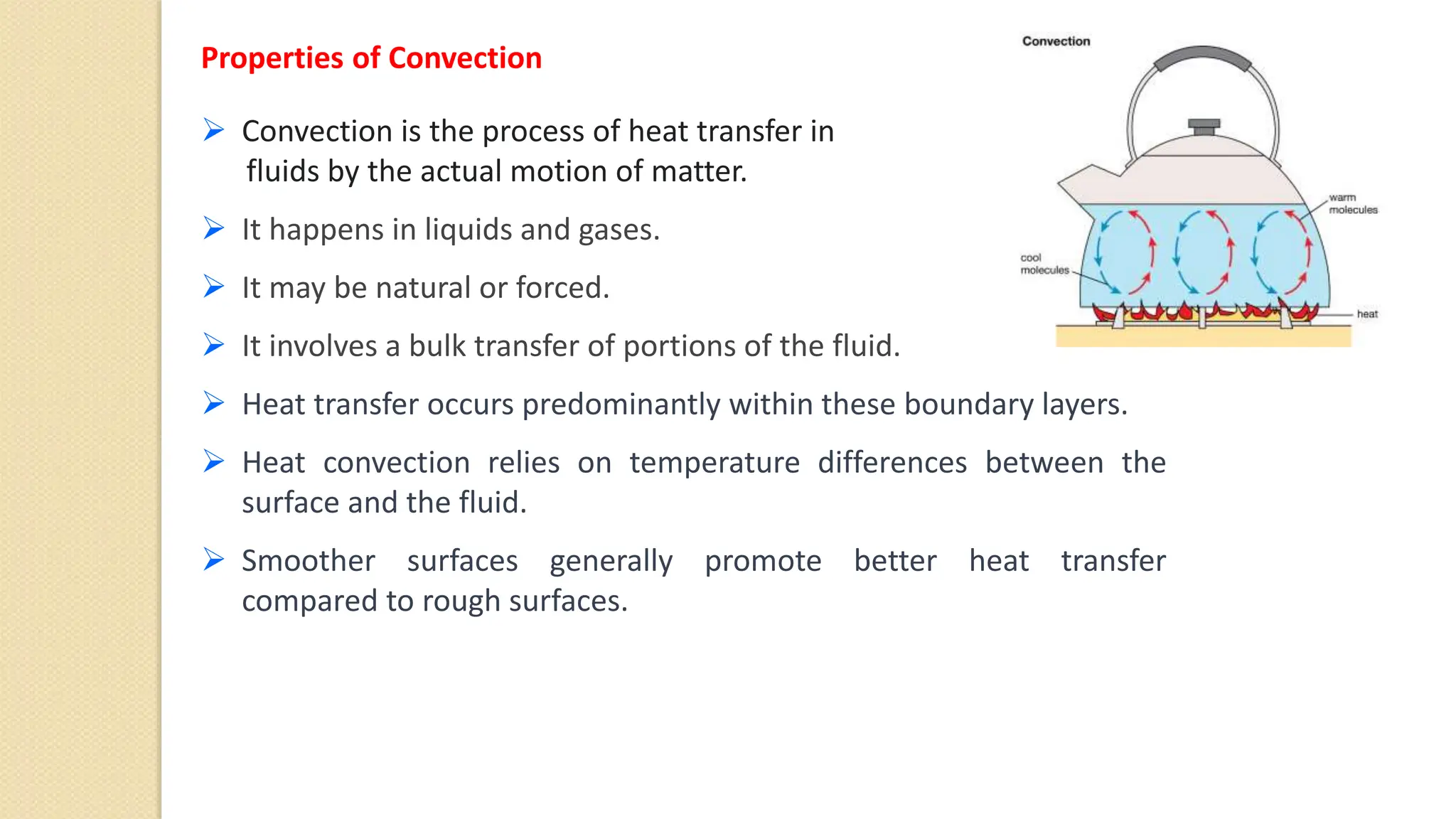
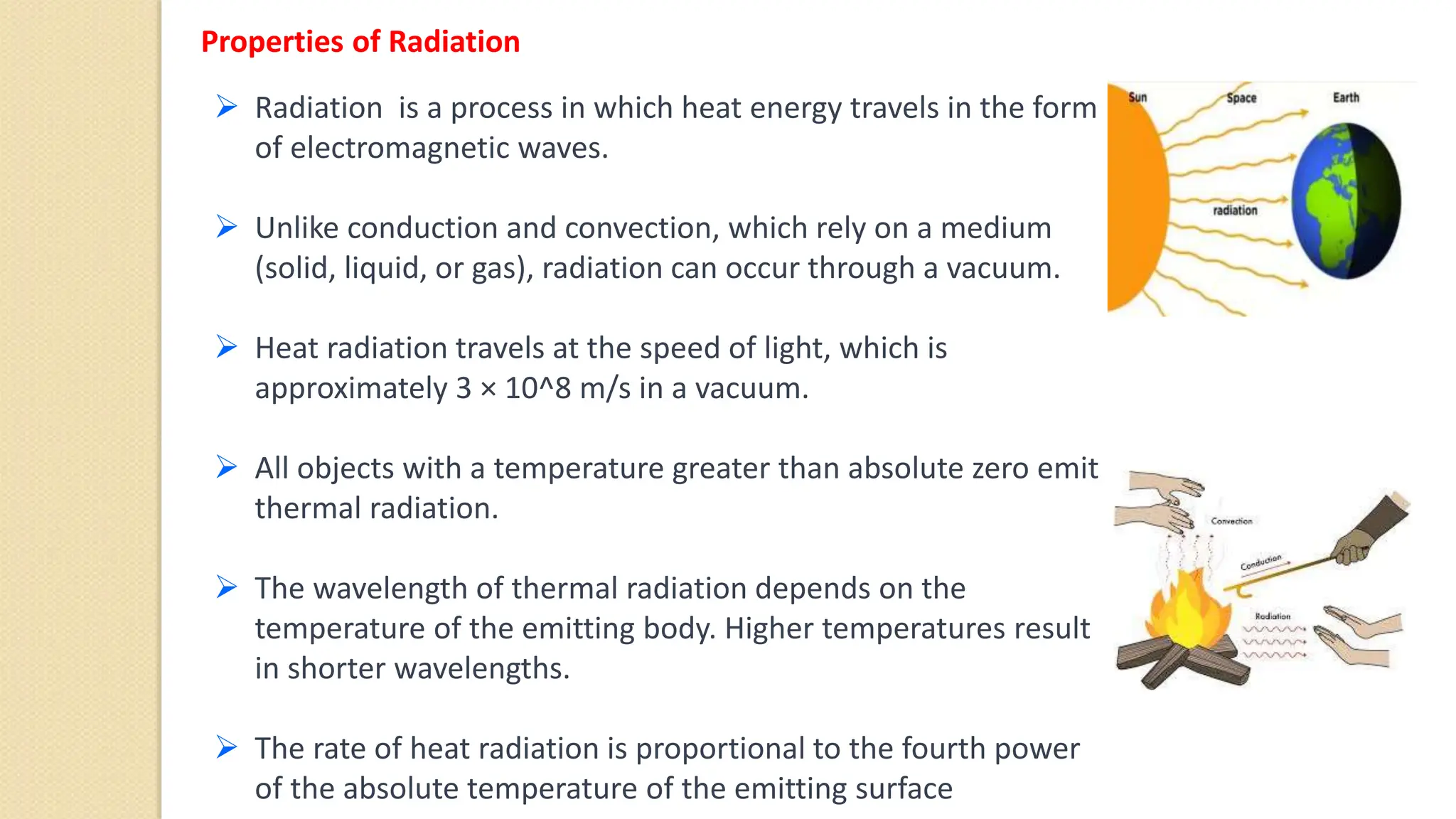
The document discusses various topics related to heat and temperature including:
1. Heat is defined as the total energy of an object from molecular motion. Temperature is a measure of the average thermal energy of particles in an object or system.
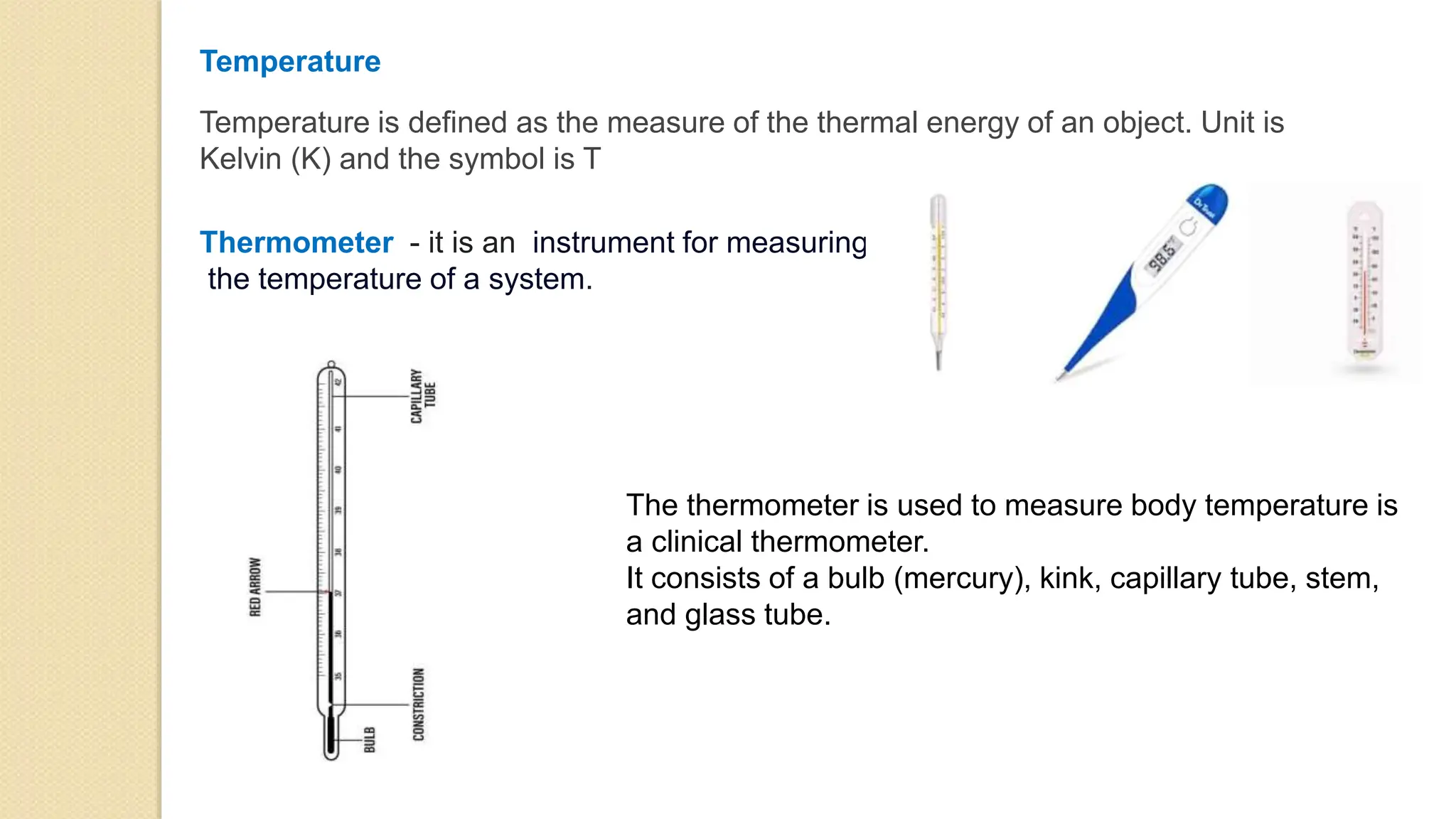
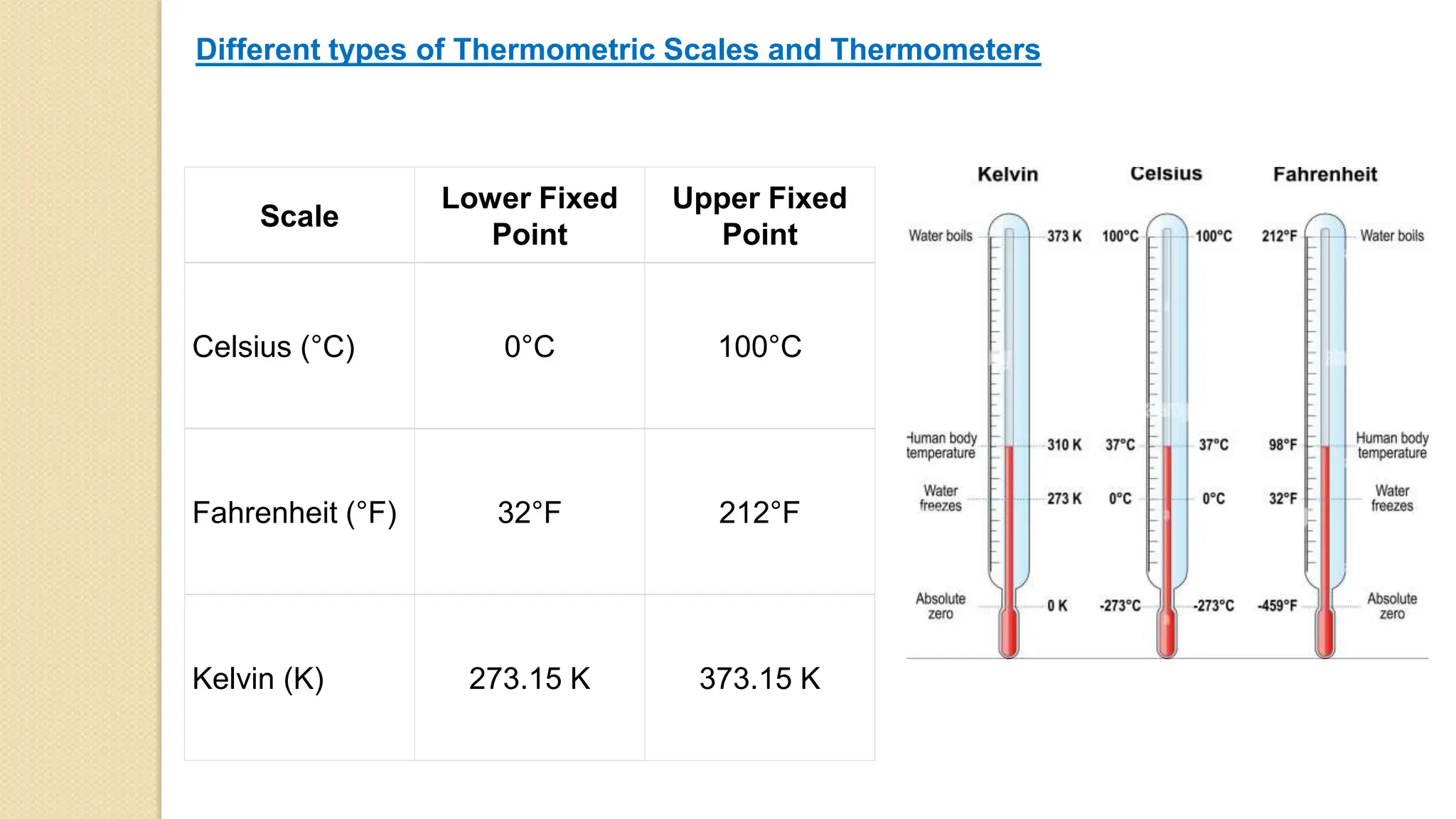
2. Common sources of heat include the sun, chemical reactions, electricity, and nuclear reactions. Thermometers are used to measure temperature using scales like Celsius, Fahrenheit and Kelvin.
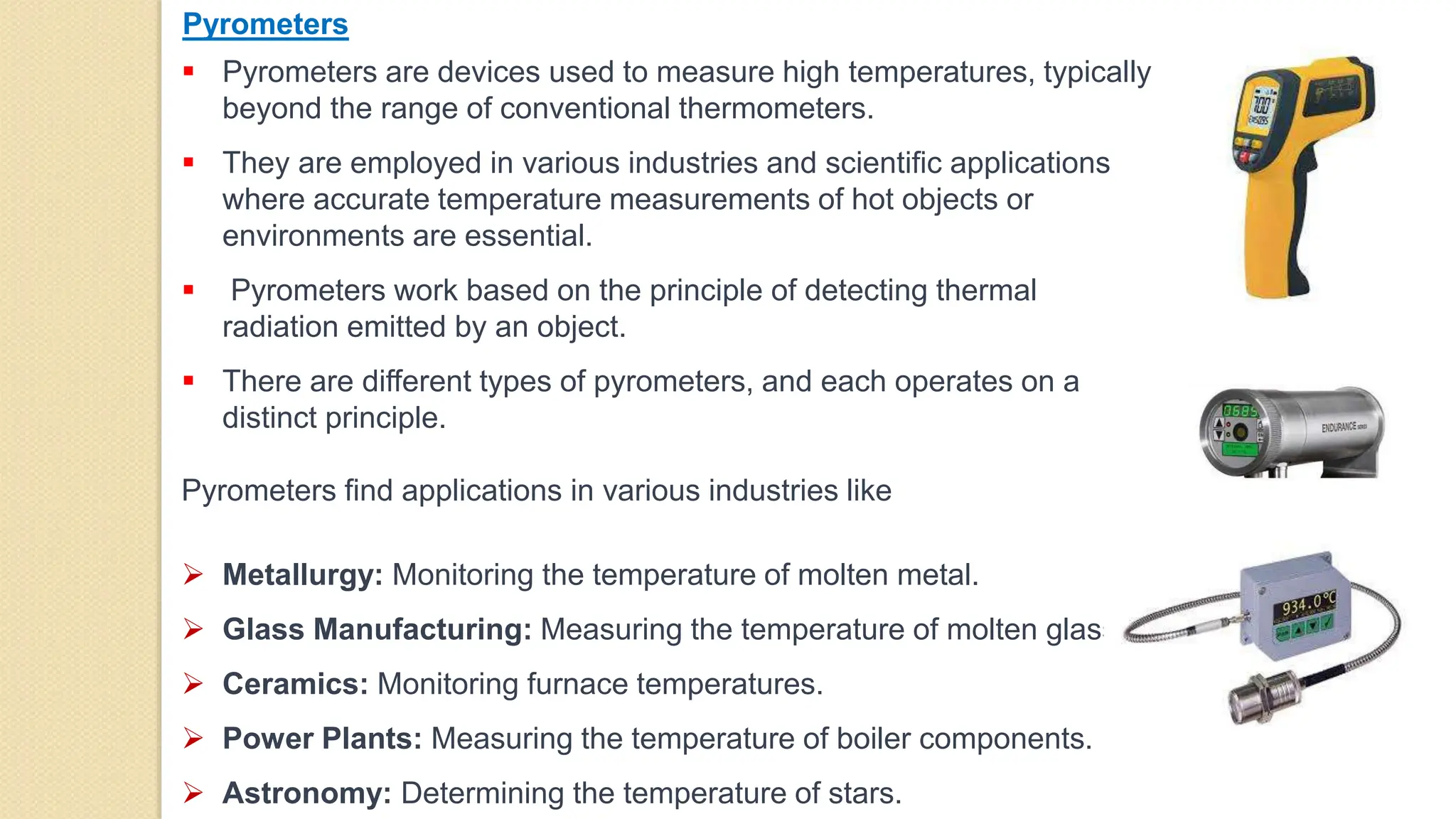
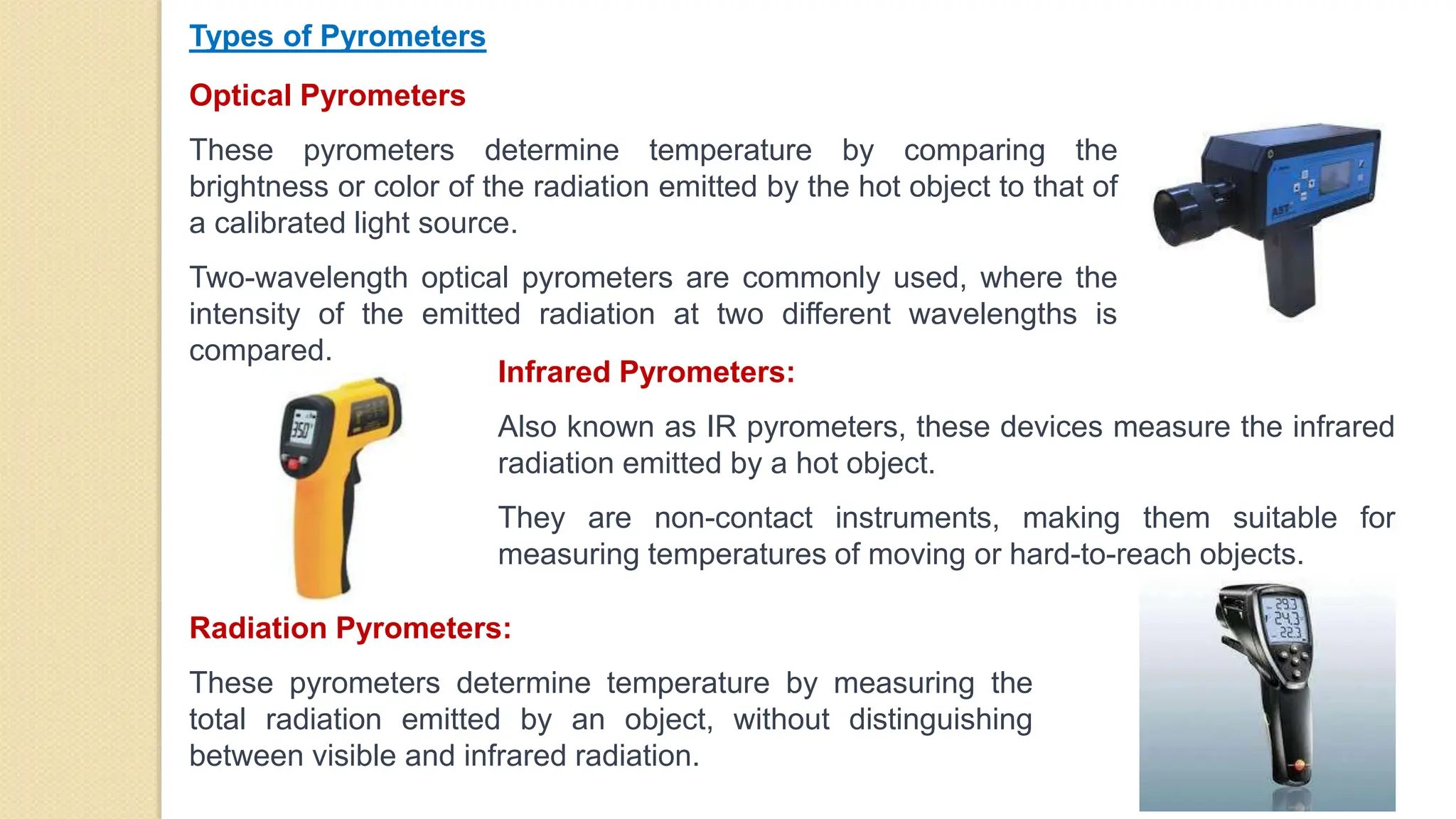
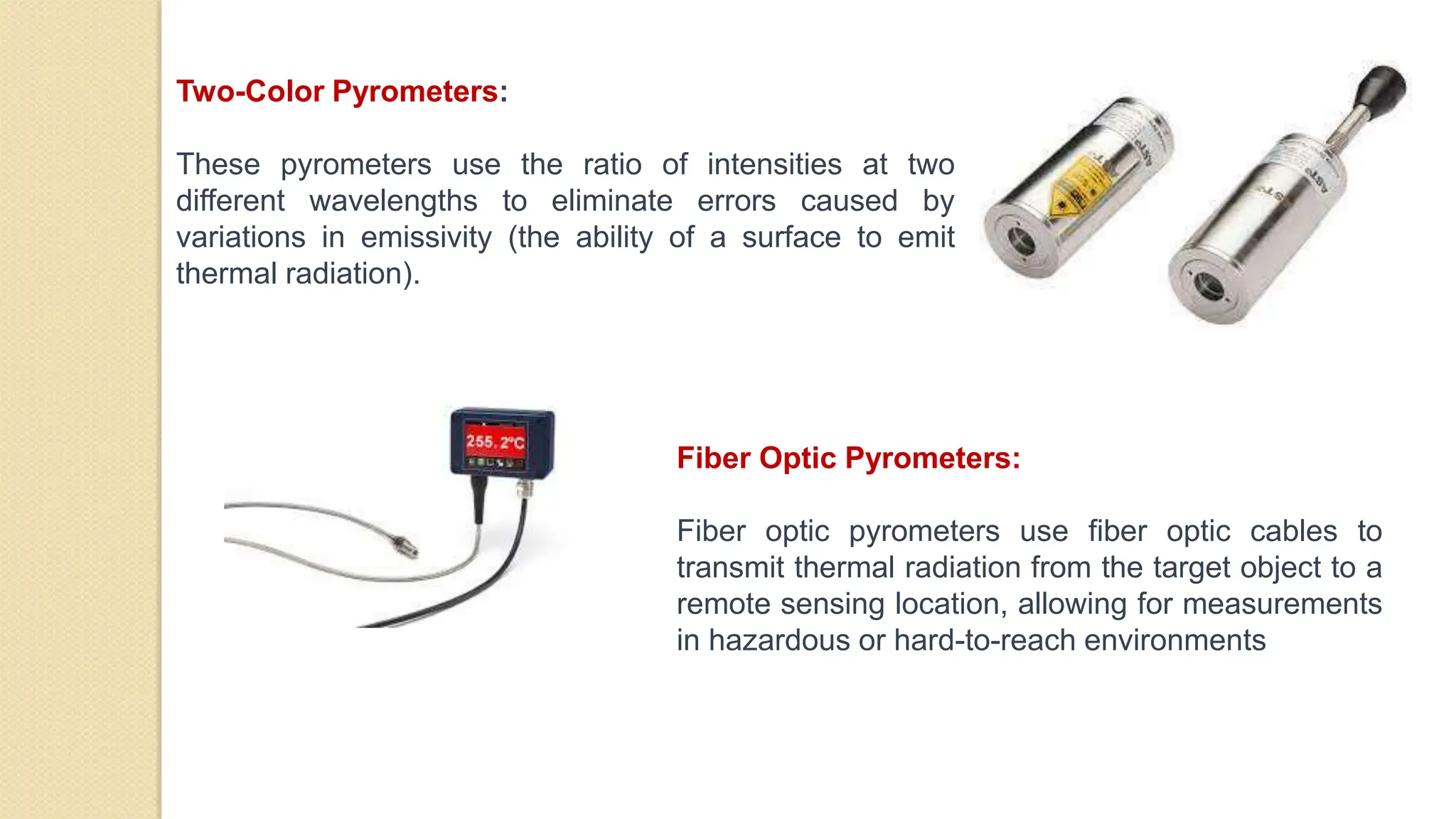
3. Pyrometers are used to measure high temperatures beyond normal thermometer ranges and work by detecting thermal radiation. Different types of pyrometers use various detection principles.
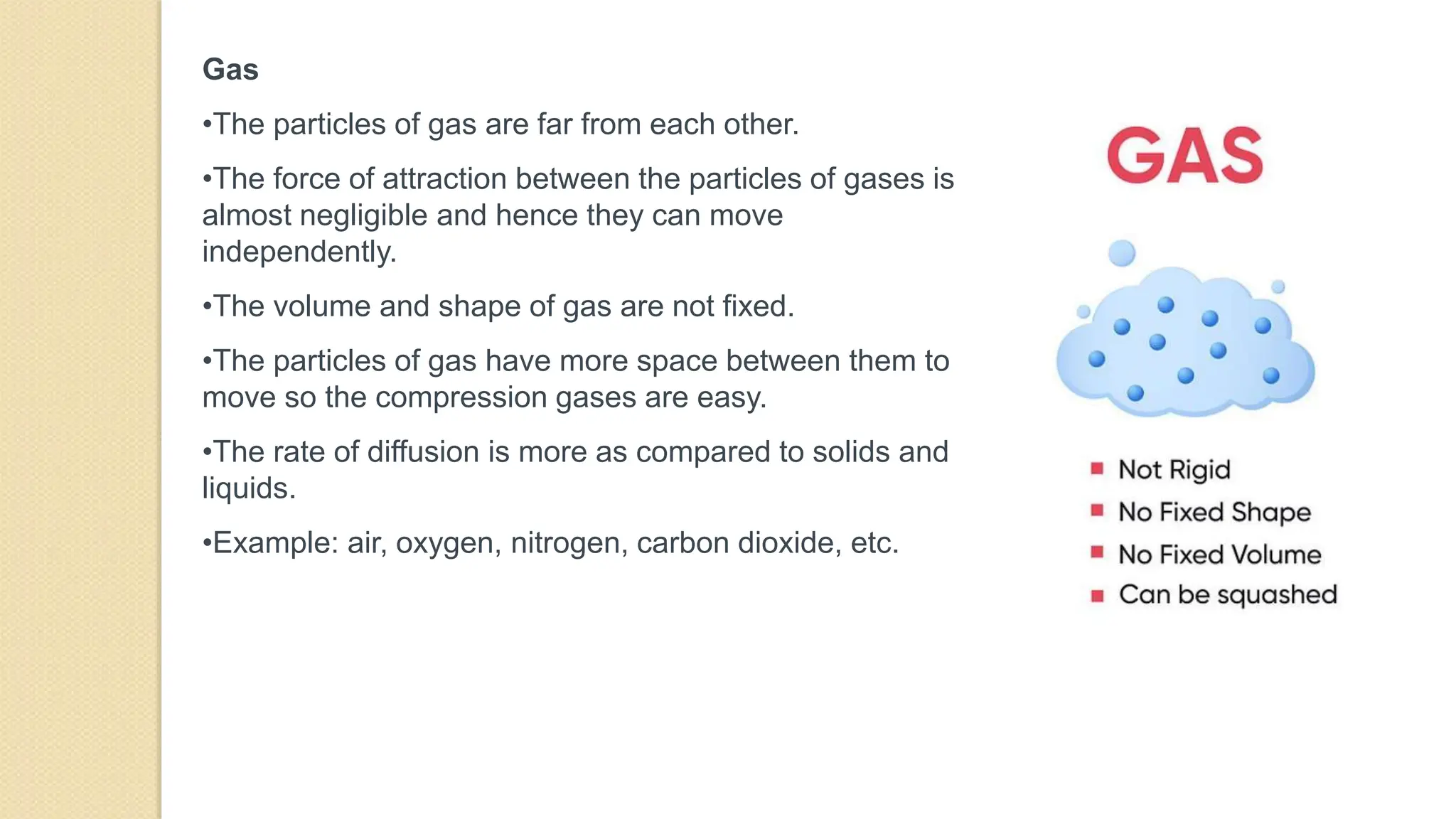
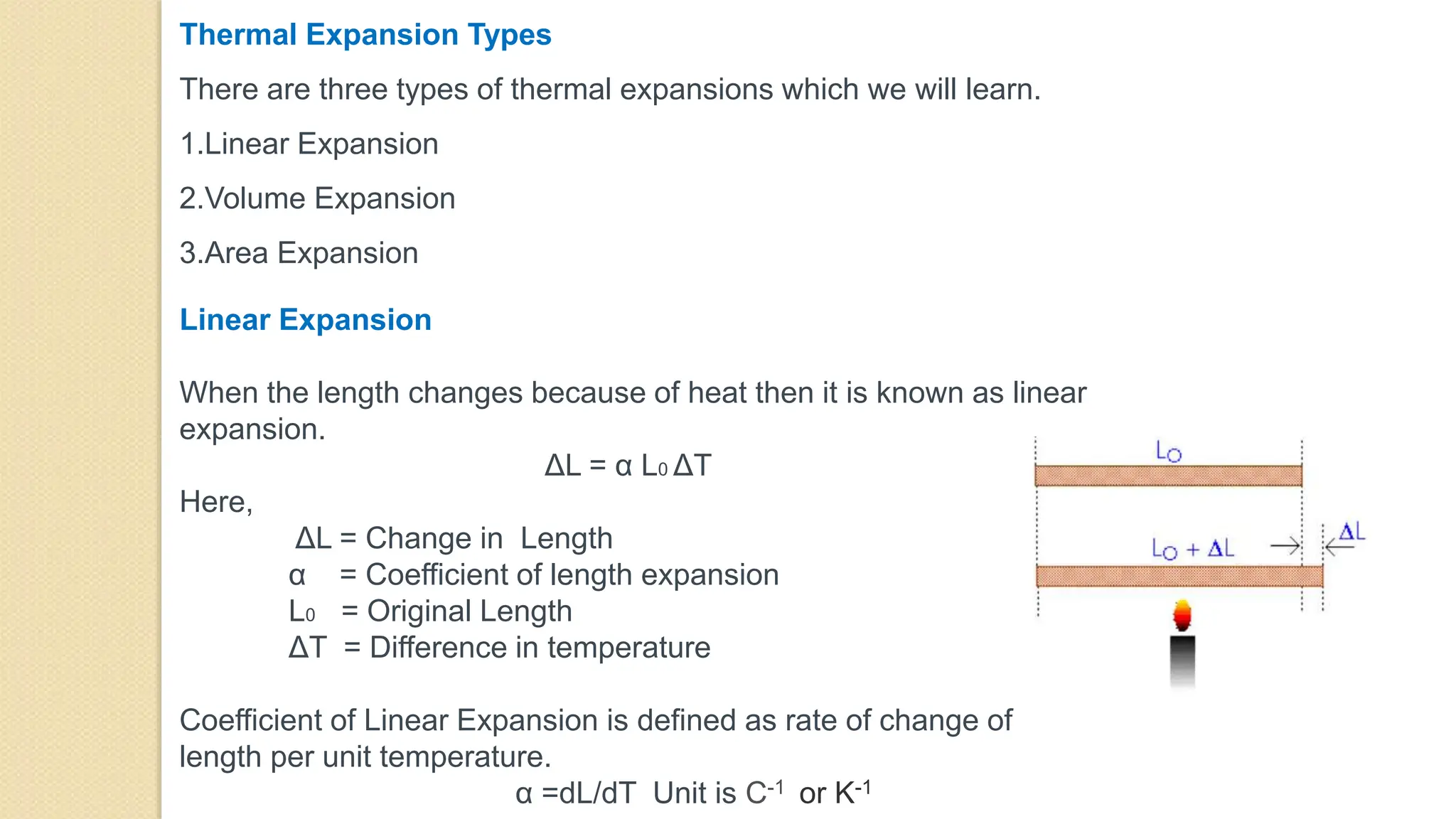
4. Thermal expansion, conduction, convection, radiation are different ways that heat is transferred. Phase