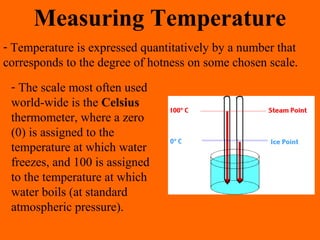
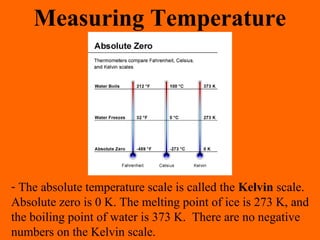
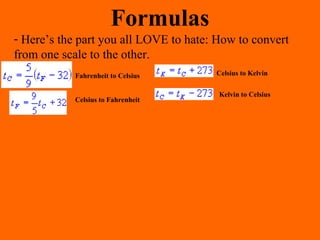
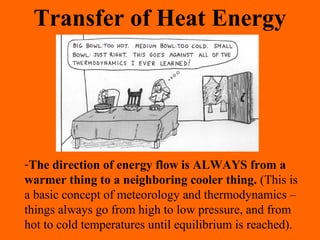
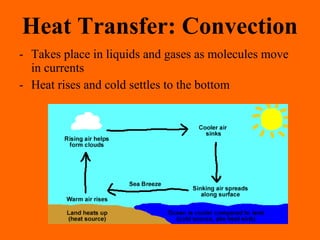
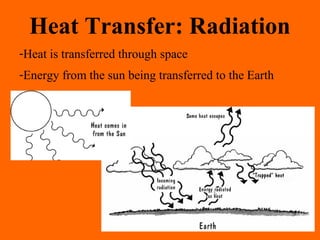
1. Heat is the transfer of thermal energy between objects due to a temperature difference, while temperature is a measure of the average kinetic energy of particles.
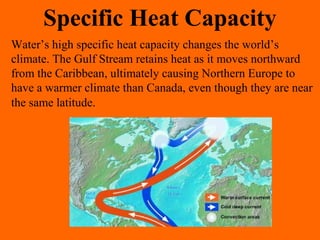
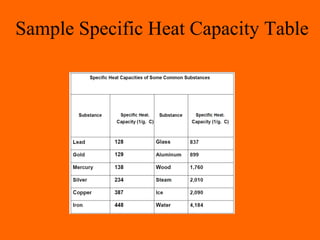
2. Specific heat capacity is the amount of heat required to change the temperature of a substance by 1°C, with water having a higher specific heat capacity than most materials.
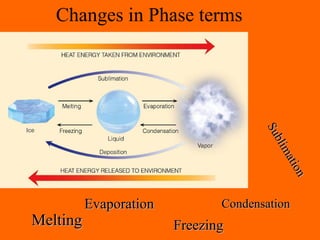
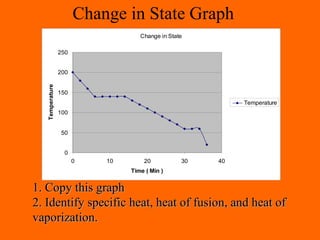
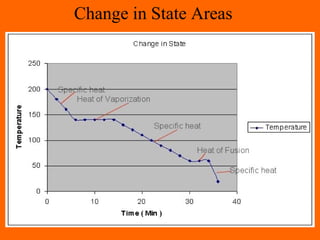
3. Phase changes from solid to liquid or liquid to gas require heat in the form of latent heat without changing temperature.