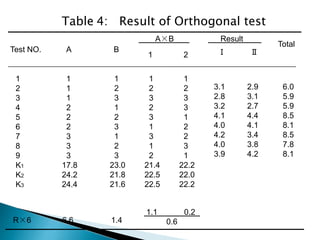
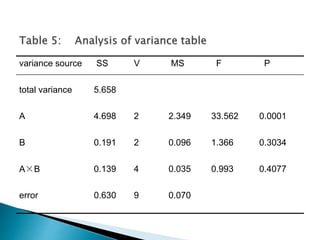
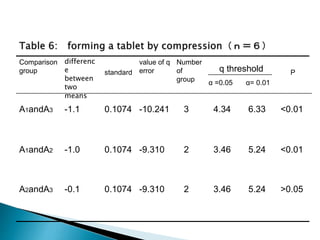
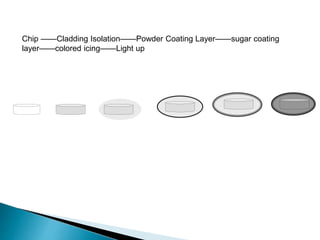
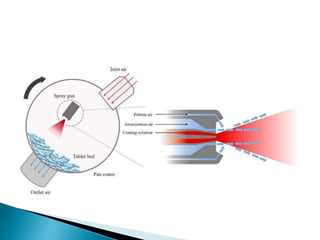
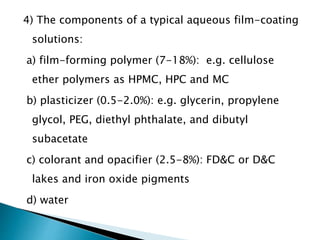
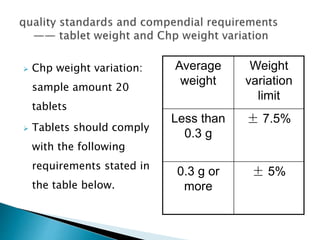
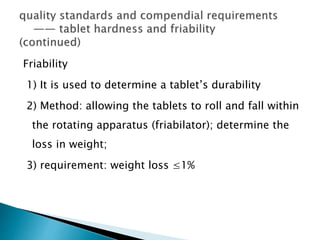
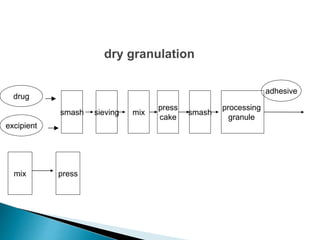
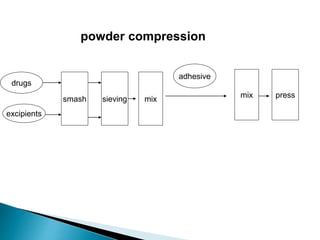
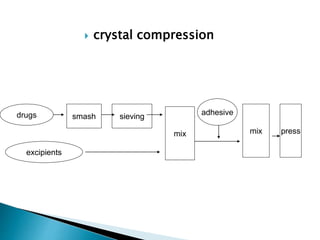
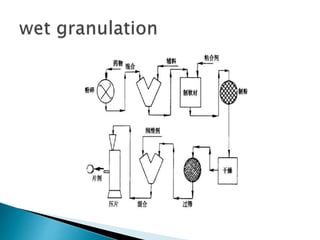
The document discusses tablets as solid dosage forms comprised of active ingredients and excipients, highlighting their classification, production methods, and various types. It details the roles and selection of excipients in tablet formulation, including binders, lubricants, and disintegrants, as well as the processes involved in tablet manufacturing such as wet and dry granulation. Additionally, it covers tablet coating methods, their purposes, and issues related to tablet quality, emphasizing the importance of various formulation factors.

















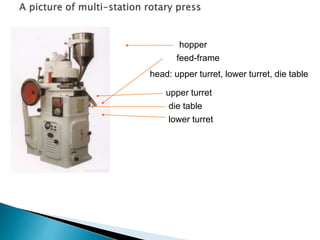

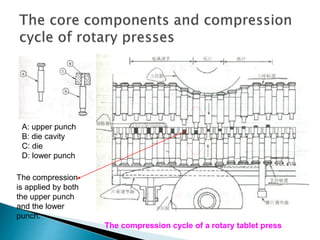




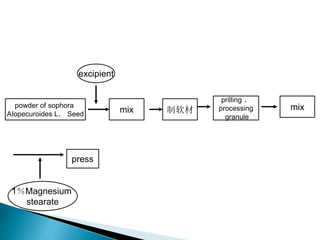
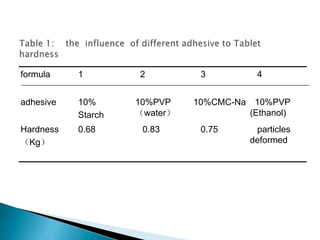
![Factor level
A [The amount of Microcrstalline
cellulose(g)]
80 120 160
A [Concentration of PVP
solution(%,g/ml)]
10 15 20](https://image.slidesharecdn.com/tabletmanufacturingtechnique-230714100349-1239ea73/85/Tablet-Manufacturing-Technique-ppt-39-320.jpg)