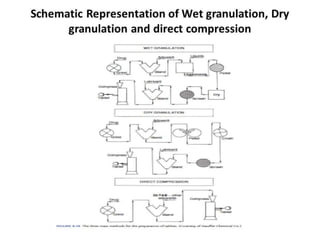
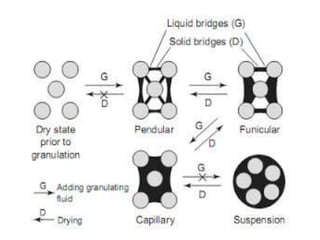
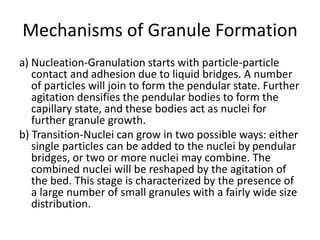
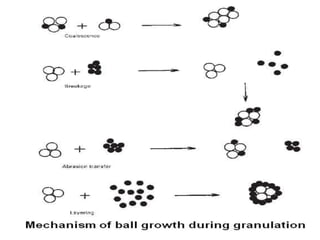
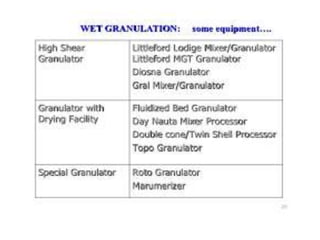
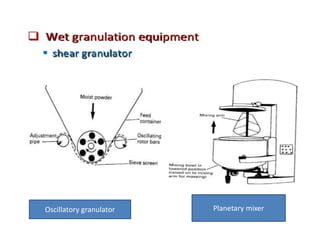
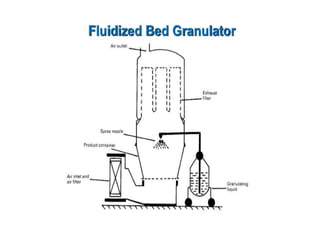
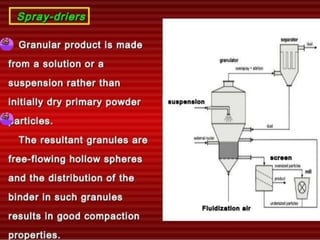
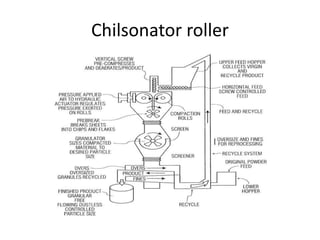
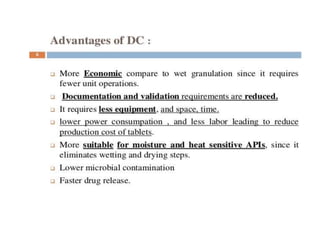
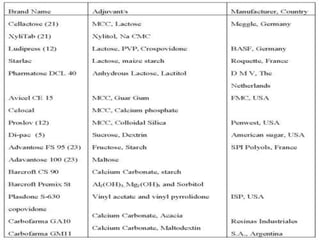
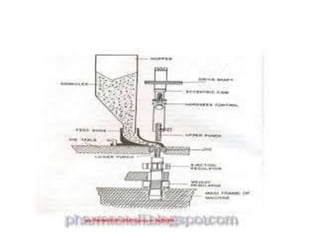
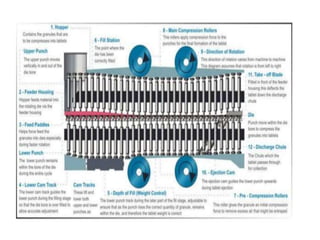
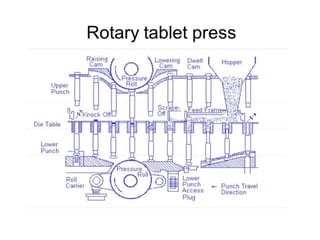
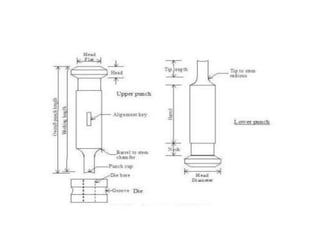
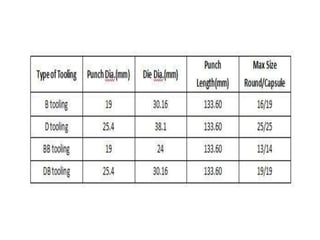
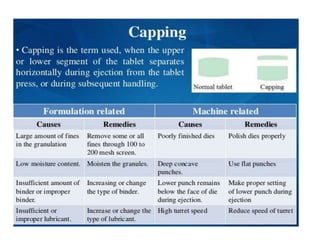
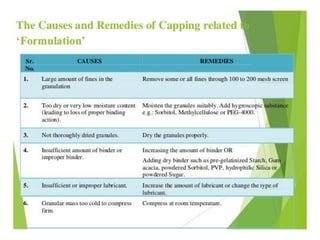
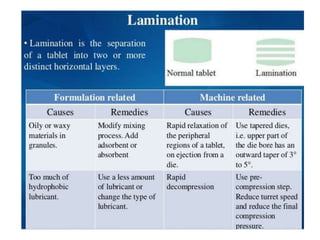
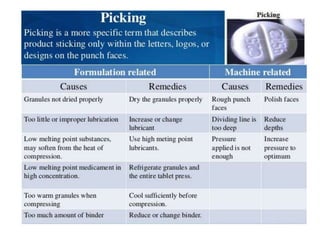
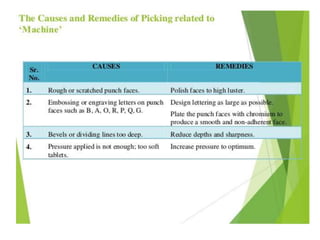
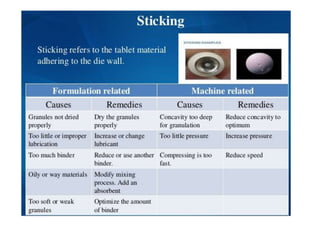
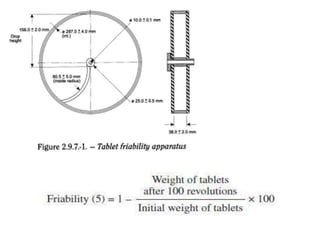
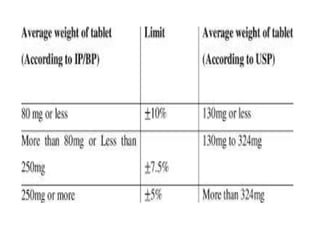
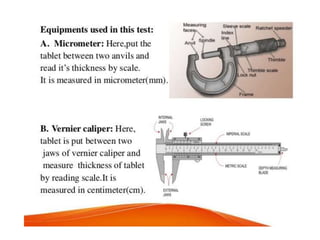
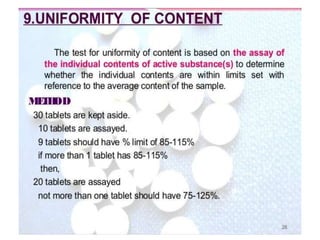
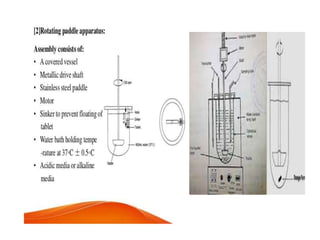
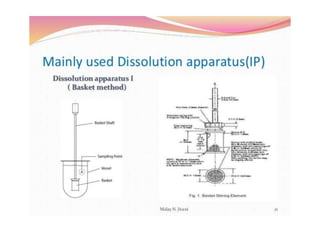
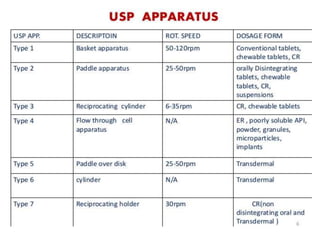
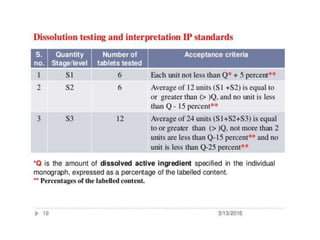
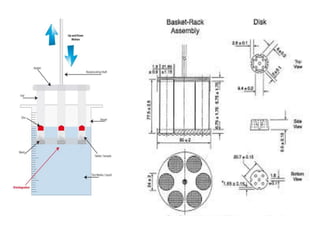
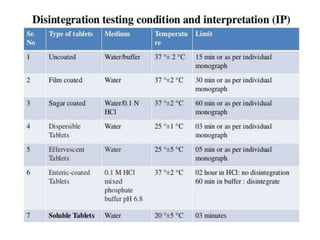
The document discusses tablets as a popular compressed solid dosage form containing medicaments, emphasizing their advantages such as precise dosing and cost-effectiveness, while also noting disadvantages like difficulty swallowing for certain patient groups. It outlines the general properties of tablets, types, ingredients, and the role of additives like diluents, binders, and disintegrants. Additionally, it addresses the granulation process, mechanisms of particle interaction, and common defects in tableting.