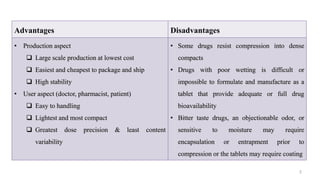
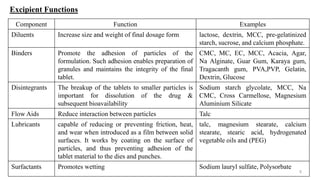
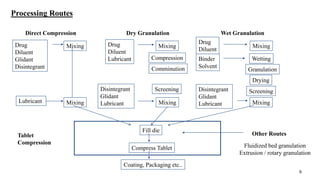
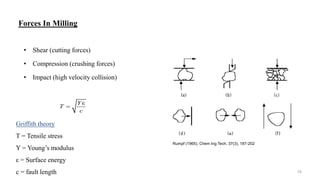
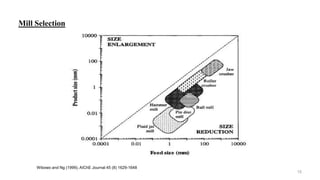
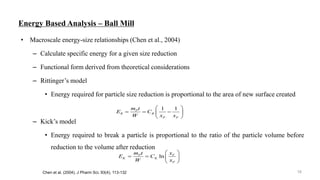
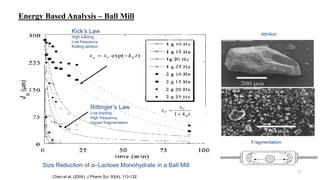
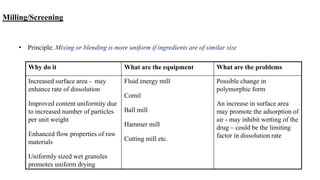
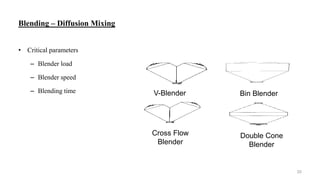
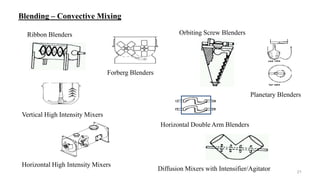
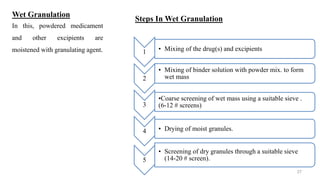
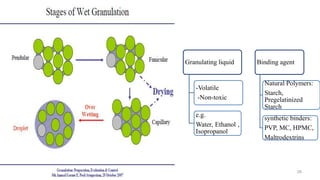
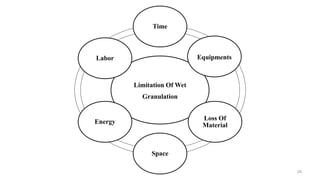
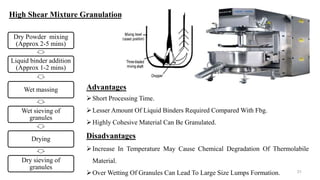
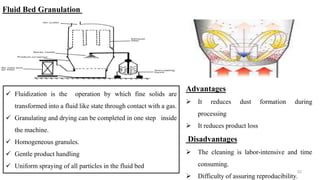
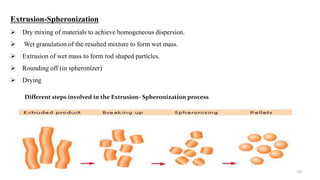
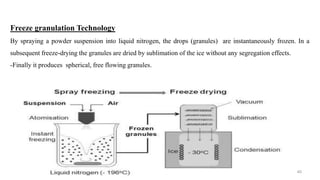
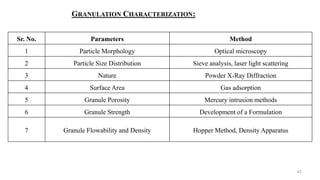
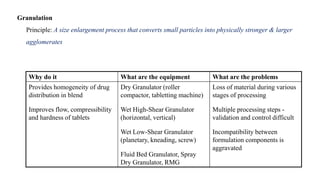
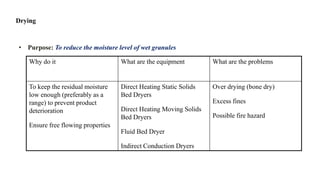
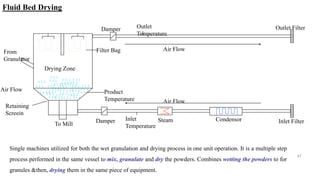
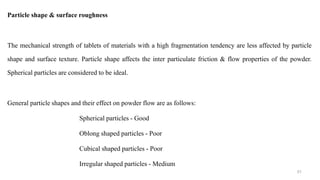
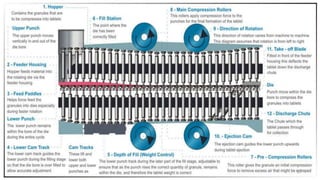
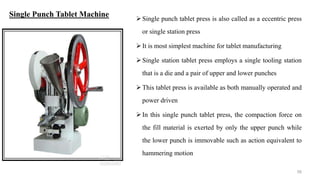
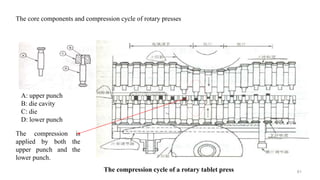
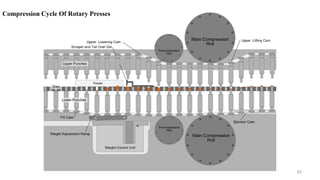
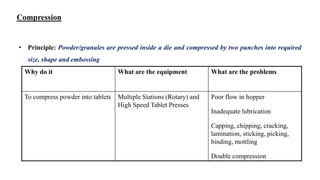
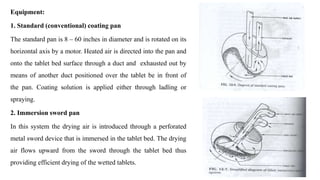
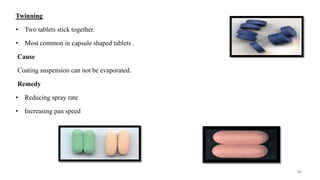
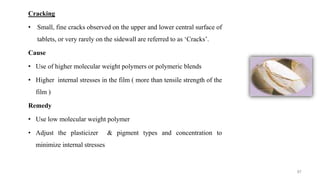
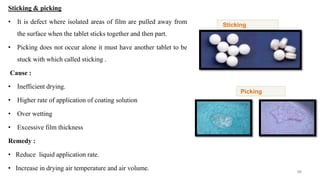
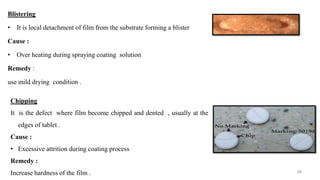
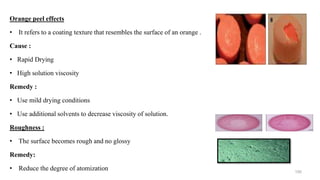
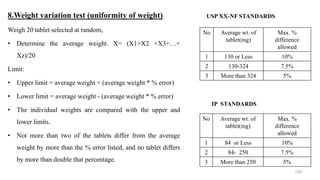
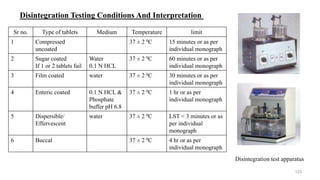
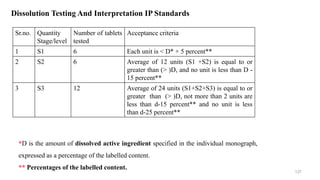
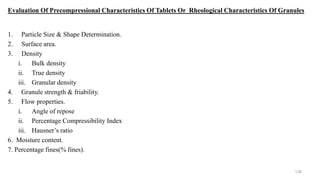
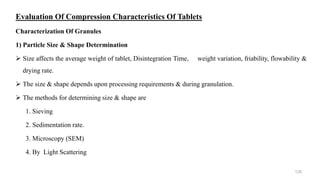
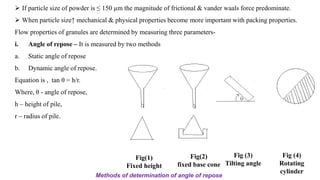
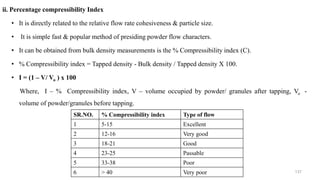
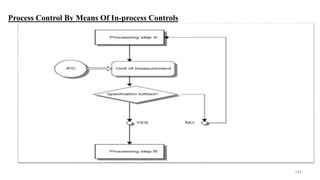
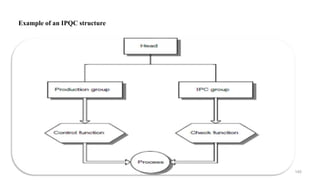
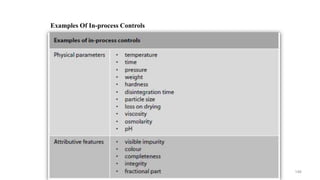
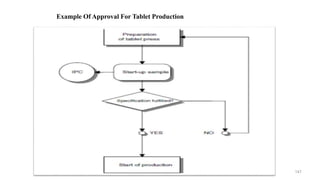
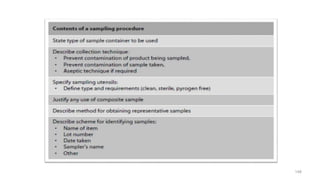
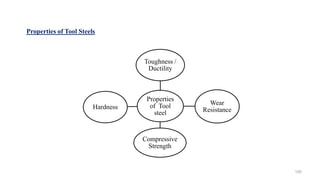
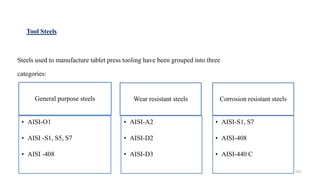
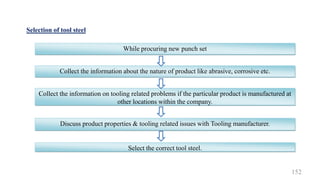
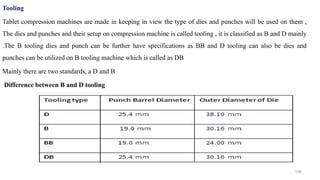
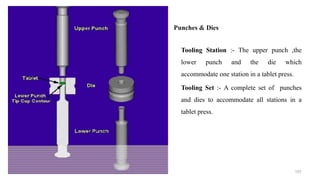
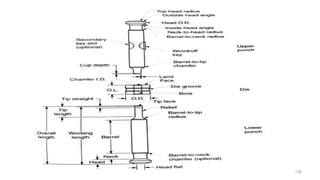
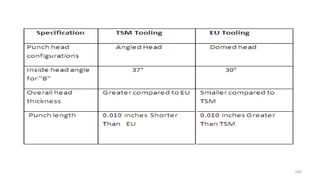
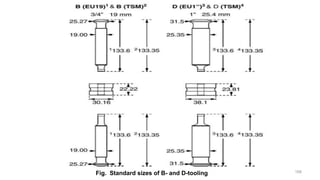
The document discusses the manufacturing processes of tablets, highlighting their advantages, types, and the role of excipients. It covers the step-by-step operations involved in tablet production, including dispensing, milling, blending, granulation, and compression, as well as various granulation techniques like wet and dry granulation. Additionally, it addresses the importance of particle size, flowability, and uniformity in achieving high-quality pharmaceutical products.