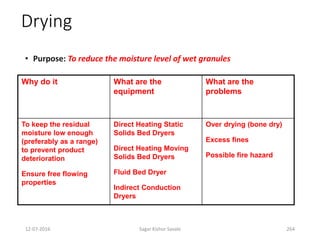
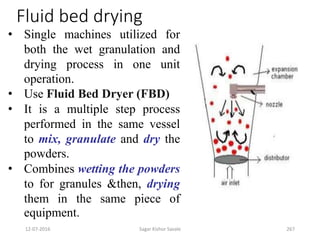
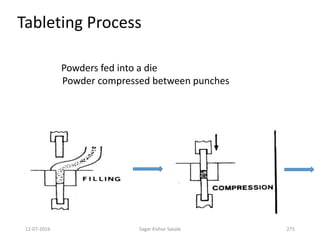
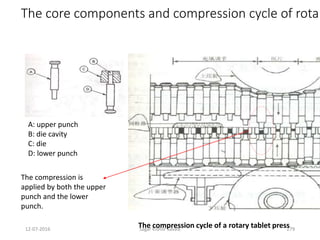
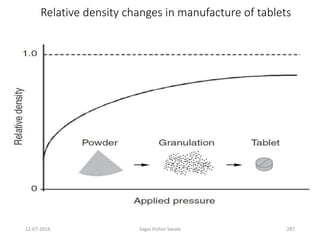
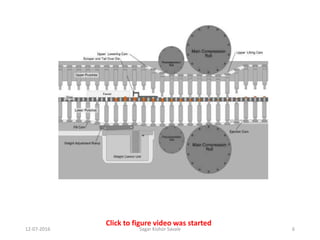
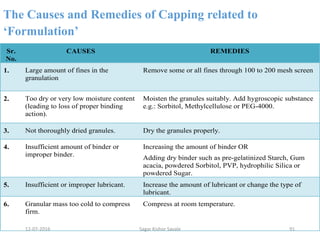
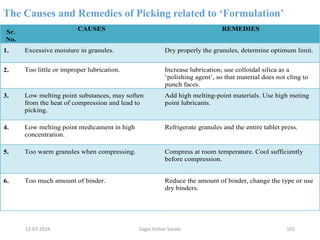
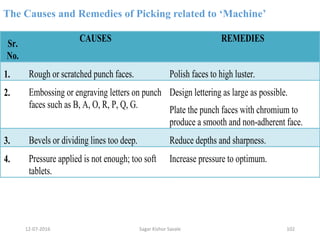
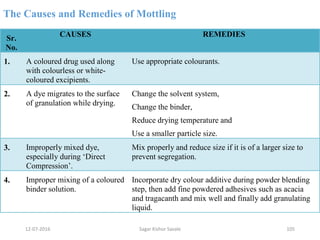
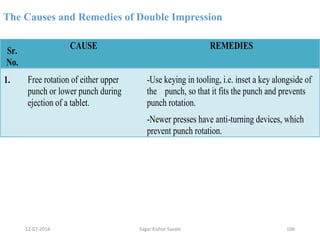
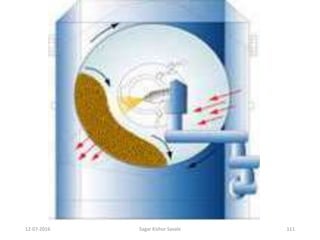
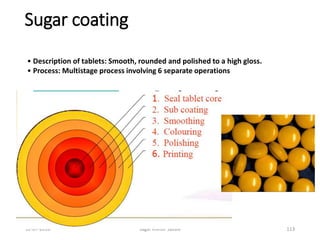
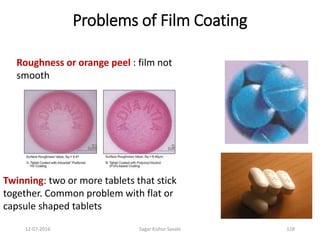
This document provides information about tablets, including their definition, advantages, types, and manufacturing process. It begins with definitions of tablets from pharmacopoeias and discusses how they are the most popular dosage form, comprising 70% of pharmaceutical preparations. It describes various types of tablets including compressed, sugar-coated, film-coated, enteric-coated, and effervescent tablets. The document outlines the tablet manufacturing process using tableting machines and discusses characteristics and specifications of compressed tablets.
![TABLETS
Mr. Sagar Kishor Savale
[Department of Pharmaceutics]
avengersagar16@gmail.com
2015 - 016
Department of Pharmacy (Pharmaceutics) | Sagar savale
112-07-2016 Sagar Kishor Savale](https://image.slidesharecdn.com/tablets-160712094157/85/Tablets-1-320.jpg)























































































































































































![Considerations
Theoretical quantity of API [100% assay (anhydrous) and nil water] = 30 Kg
22212-07-2016 Sagar Kishor Savale](https://image.slidesharecdn.com/tablets-160712094157/85/Tablets-222-320.jpg)







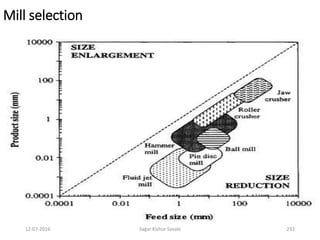







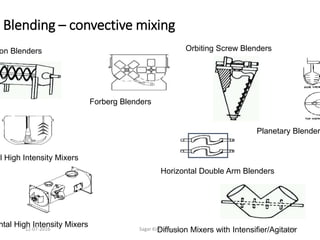
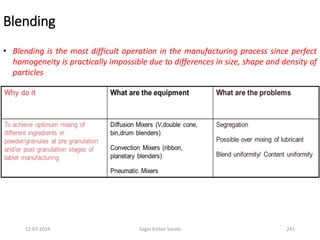













![12-07-2016 Sagar Kishor Savale 259
Recent Advances in Granulation Techniques
• Steam Granulation: Modification of wet granulation; steam
is used as a binder instead of water; granules are more
spherical and exhibit higher rate of dissolution
• Melt Granulation / Thermoplastic Granulation: Granulation
is achieved by the addition of meltable binder i.e. binder is in
solid state at room temperature but melts in the
temperature range of 50 – 80˚C [e.g. PEG (water soluble),
stearic acid, cetyl or stearyl alcohol (water insoluble)] -
drying phase unnecessary since dried granules are obtained
by cooling them to room temperature
• Moisture Activated Dry Granulation (MADG): Involves
distribution of moisture to induce agglomeration – drying
time is reduced](https://image.slidesharecdn.com/tablets-160712094157/85/Tablets-259-320.jpg)