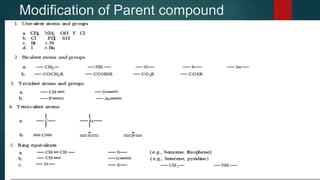
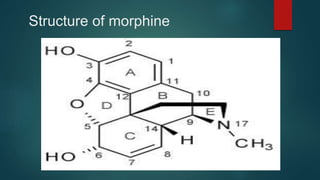
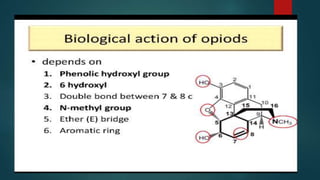
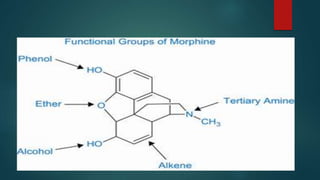
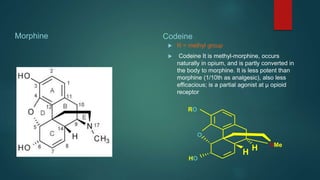
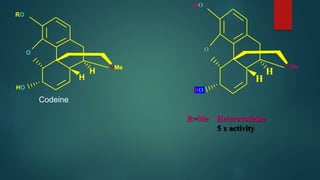
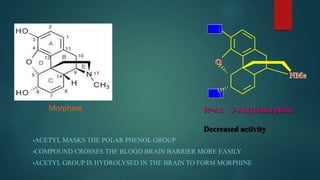
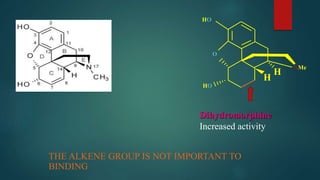
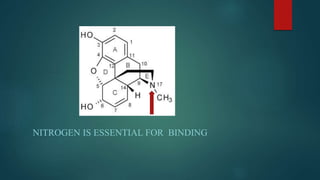
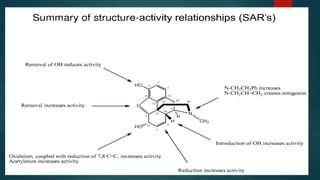
The document discusses the structure-activity relationship (SAR) of opioids. It begins by defining SAR as the relationship between a molecule's chemical structure and its biological activity. Modifications to a drug's structure, including altering functional groups, can change its potency and effects. The structures of morphine, codeine, and heroin are examined as examples. While the methyl group on codeine decreases potency versus morphine, the acetyl groups on heroin allow it to cross the blood-brain barrier more easily and produce a stronger euphoric effect. Understanding SAR enables the design of new opioid drugs with tailored pharmacological properties.