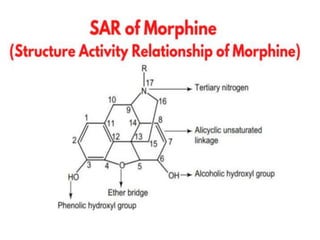
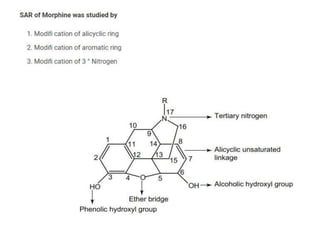
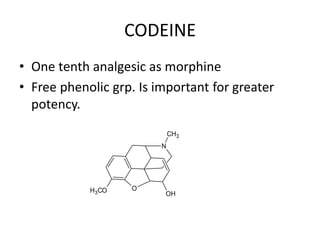
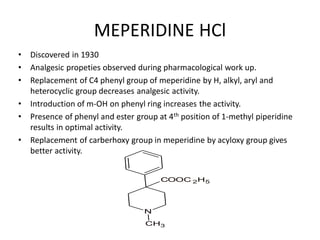
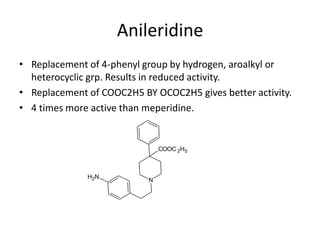
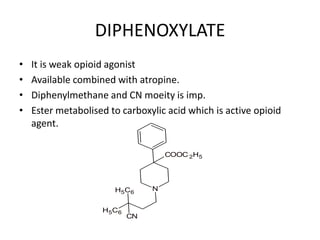
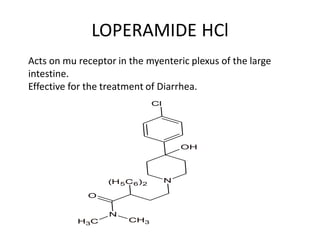
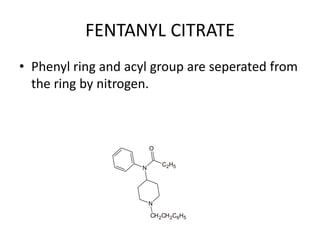
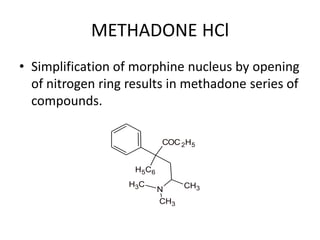
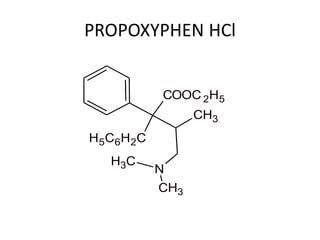
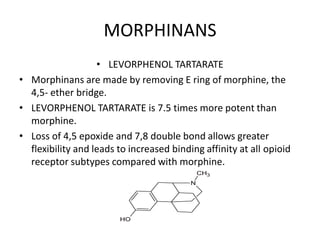
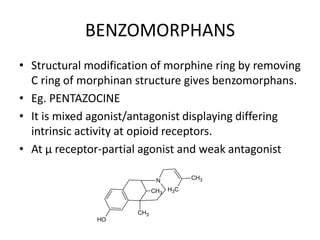
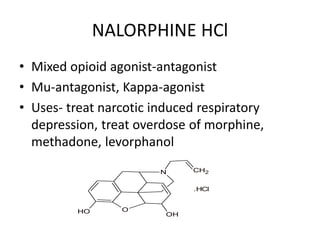
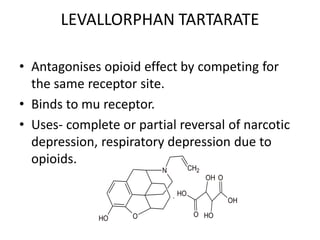
The document discusses various classes of analgesics, including opioid and non-opioid types, highlighting specific compounds such as codeine, meperidine, and fentanyl, along with their structural modifications and corresponding analgesic activities. It also covers narcotic antagonists that block the effects of opioids, detailing their mechanisms and medical applications, particularly in overdose situations. Key findings include the potency and effectiveness of modified compounds compared to traditional morphine derivatives.