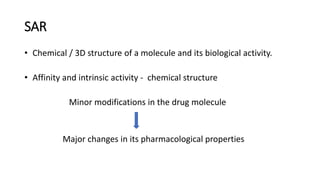
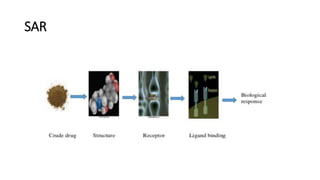
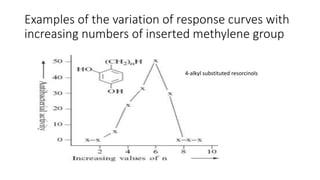
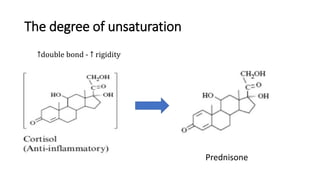
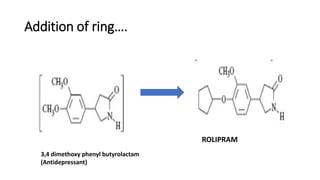
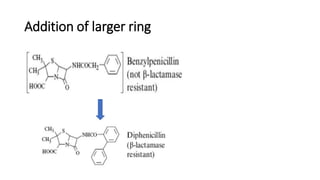
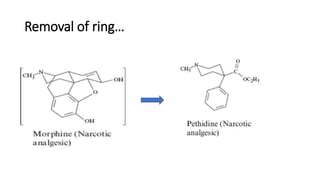
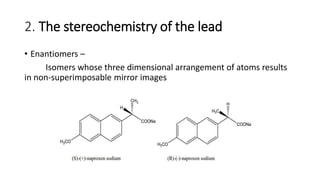
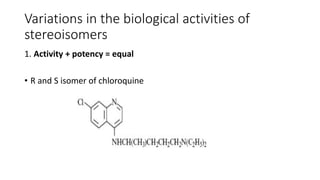
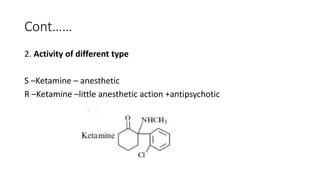
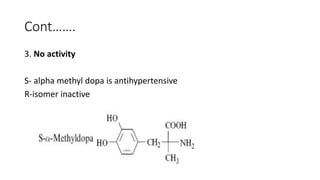
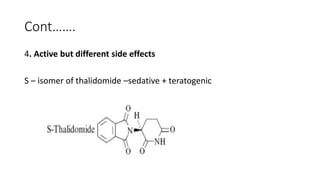
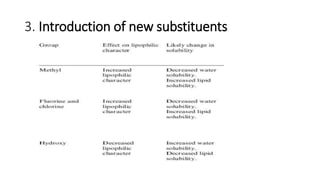
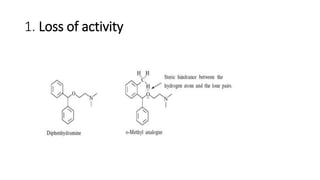
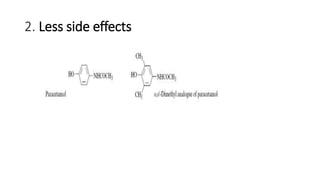
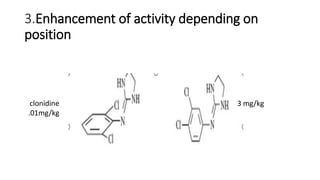
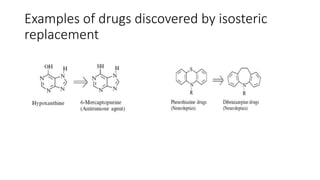
The document discusses the general principles of Structure-Activity Relationship (SAR). SAR studies how minor modifications to a drug molecule's chemical structure can lead to major changes in its pharmacological properties. SAR is used to determine a drug's pharmacophore, reduce unwanted side effects, and develop new drugs with increased activity. The key aspects of SAR covered are the size and shape of a molecule's carbon skeleton, its stereochemistry, and the nature and degree of substitution.