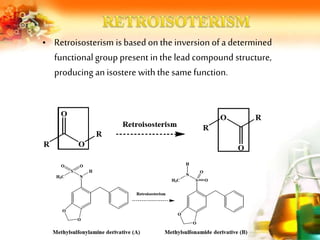
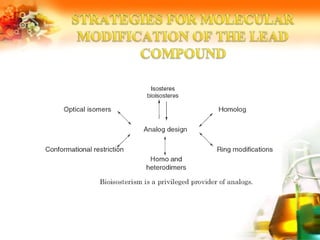
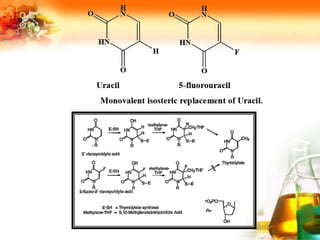
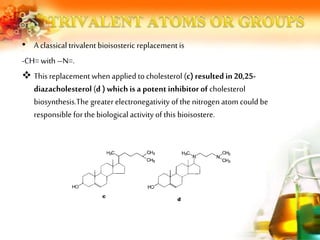
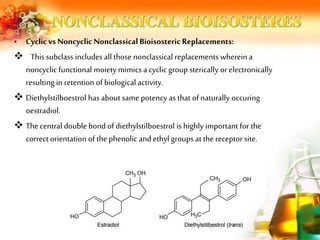
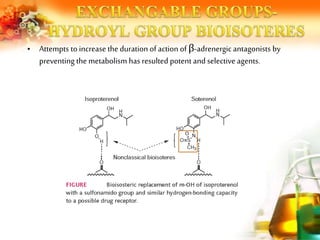
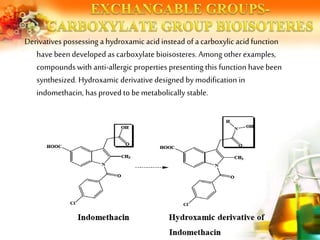
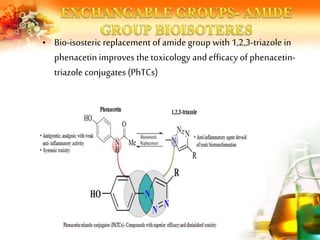
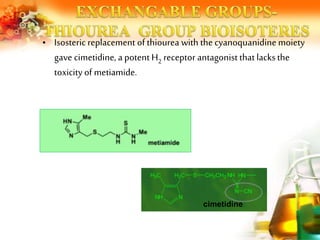
The document presents an overview of analog design and bioisosterism in drug development, highlighting the modification of drug molecules to enhance therapeutic properties while minimizing adverse effects. It categorizes bioisosteres into classical and non-classical types and provides examples of chemical substitutions that have led to effective drug analogs. The synthesis of bioisosteres is emphasized as a crucial strategy in improving pharmacological outcomes through subtle changes in physicochemical properties.









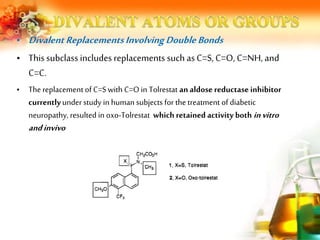




![• Replacements of this type were observed in a series of 1-[(2- hydroxyethoxy)
methyl]-5- benzyluracils that were tested for inhibition of liver uridine
phosphorylase (UrdPase) 35.
• This hypothesis was supported by the observation that replacement of the
chloro atom with stronger electronwithdrawing groups such as the cyano or
the trifluromethyl resulted in less potent analogues](https://image.slidesharecdn.com/analogdesign-bioisosterism-210810062018/85/Analog-design-bioisosterism-23-320.jpg)