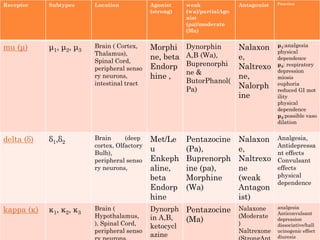
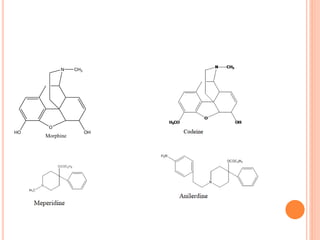
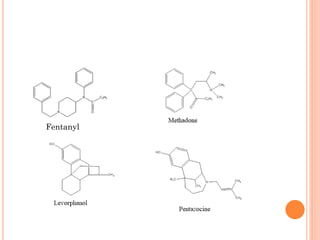
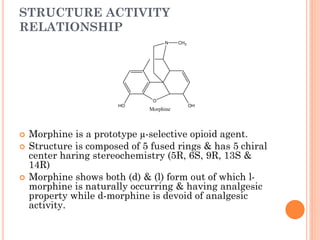
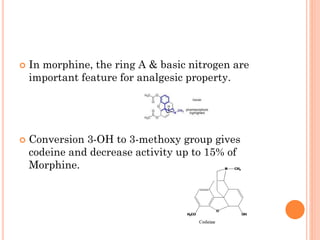
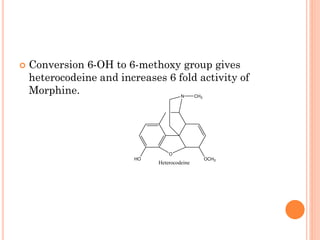
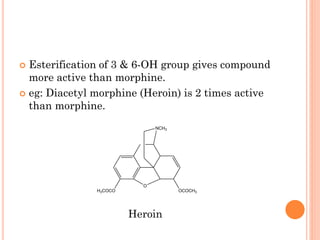
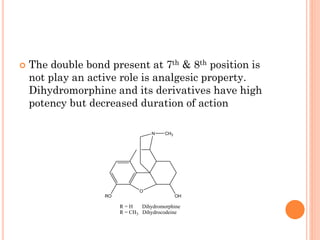
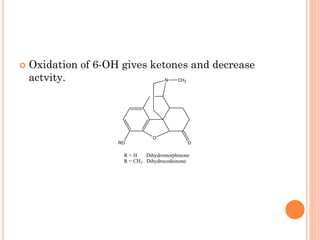
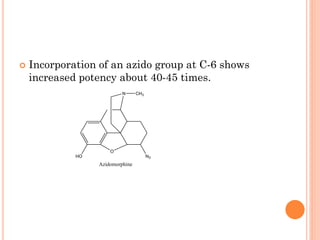
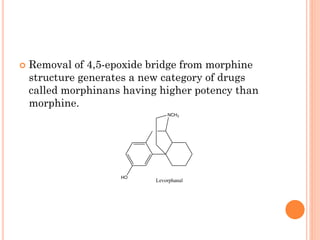
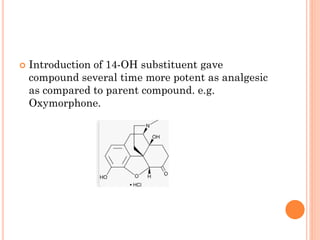
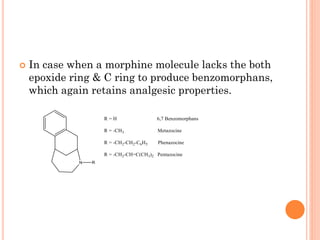
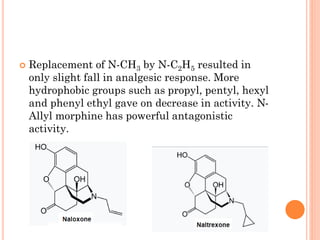
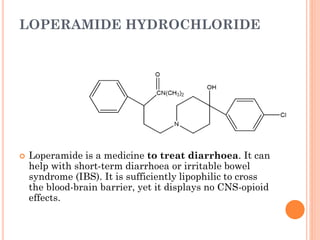
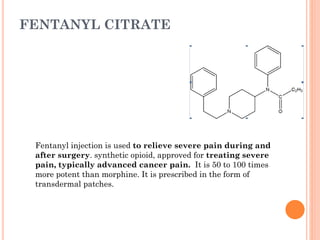
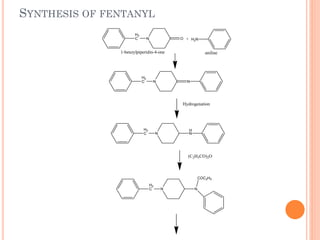
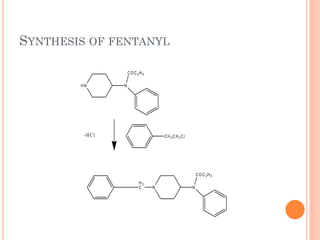
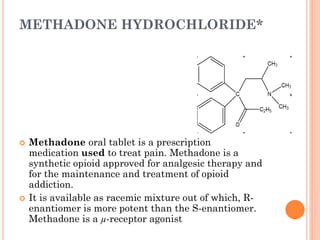
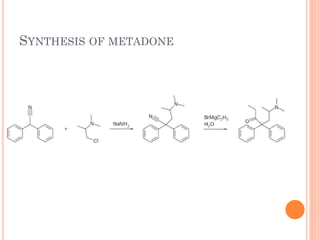
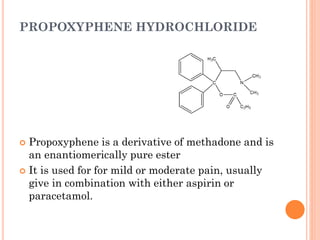
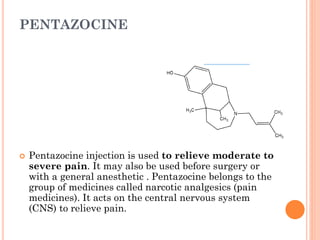
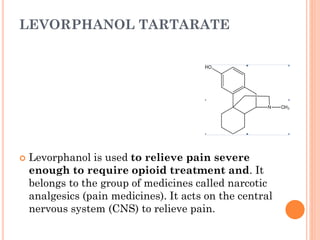
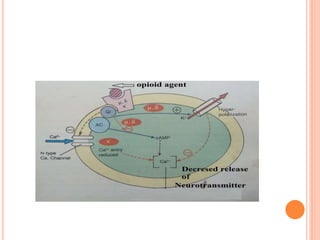
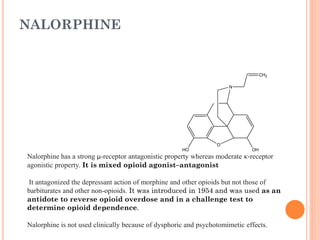
This document summarizes opioid analgesics. It discusses the classification of opioid analgesics as natural alkaloids like morphine and codeine, semisynthetic drugs, and synthetic opioids. It describes the structures and actions of various opioid analgesics like morphine, codeine, fentanyl, methadone, and antagonists like naloxone. It also discusses the modes of action of opioid analgesics through opioid receptor subtypes and their effects in reducing neurotransmitter release.