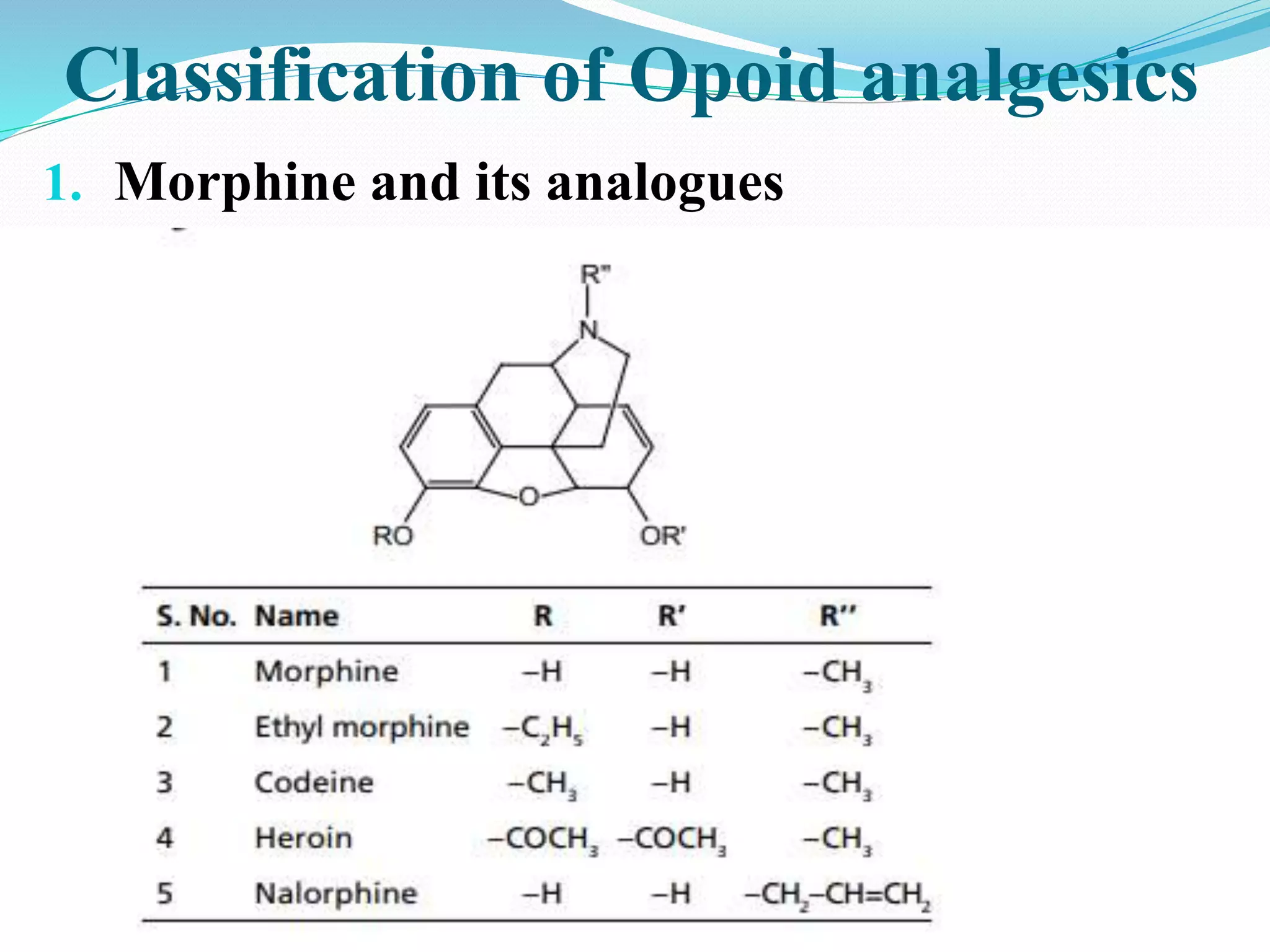
The document discusses analgesics, defining them as agents that relieve pain without affecting consciousness and classifying them into opioid and non-opioid types. It details the properties and effects of opioid analgesics, including their mechanisms of action, potential side effects, and structure-activity relationships for various compounds like morphine and methadone. Additionally, the synthesis and specific characteristics of drugs such as fentanyl and methadone are explored.