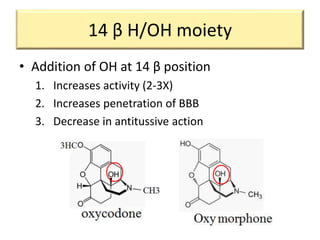
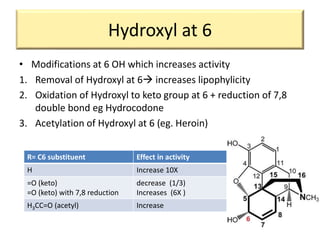
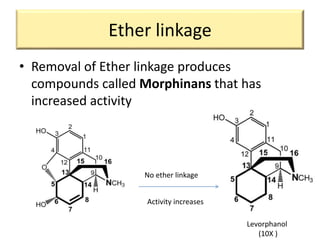
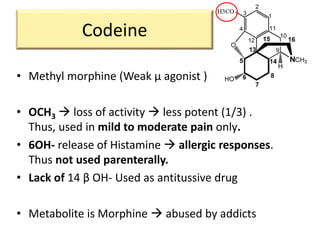
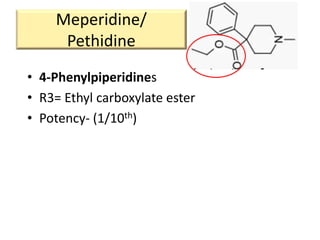
1. The biological activity of opioids depends on structural features like the phenolic hydroxyl group, the 6-hydroxyl group, the double bond between carbons 7 and 8, and the N-methyl group.
2. Modifications to these structural features can increase or decrease opioid potency and selectivity. For example, removing the 6-hydroxyl group increases lipophilicity and potency, while adding a hydroxyl group at carbon 14 increases activity and BBB penetration.
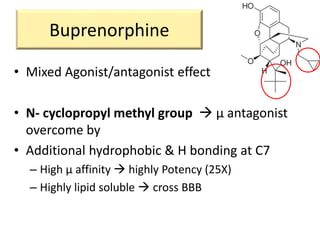
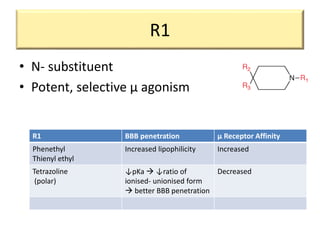
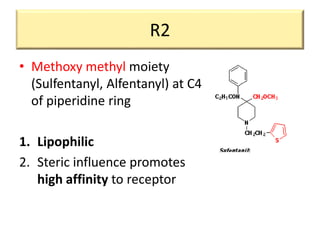
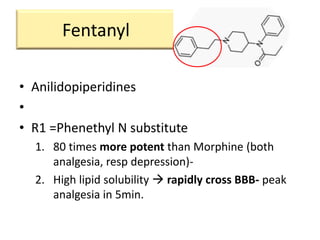
3. Different opioid subclasses like morphinans, benzomorphans, and phenylpiperidines vary in potency and efficacy depending on their ring structures and substituents on nitrogen. For example, fentanyls have high