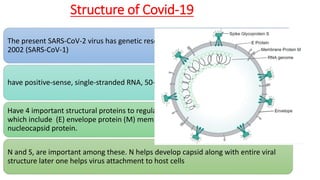
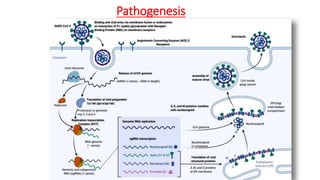
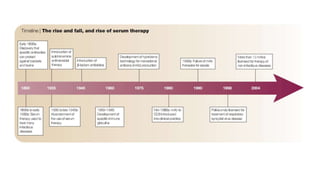
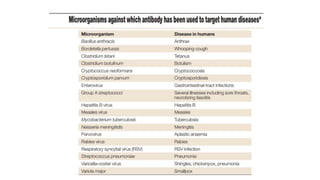
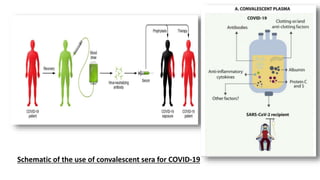
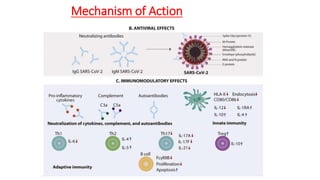
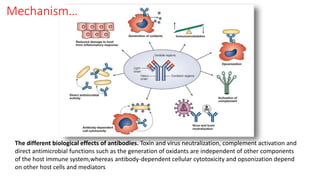
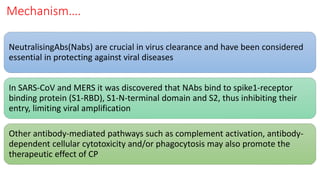
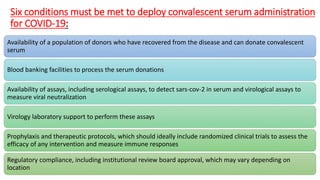
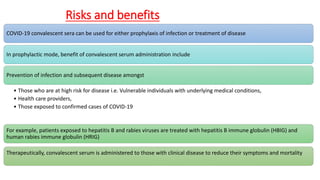
The document discusses convalescent plasma therapy as a potential treatment for COVID-19, detailing the history and mechanisms of antibody therapy, as well as criteria for donor eligibility and patient eligibility for plasma administration. It emphasizes the need for rigorous clinical trials to evaluate the efficacy and safety of convalescent plasma, while noting the known and theoretical risks associated with its use. Current research indicates improvements in patient outcomes with convalescent plasma transfusion, but highlights limitations due to a lack of large-scale studies.