Embed presentation
Downloaded 12 times
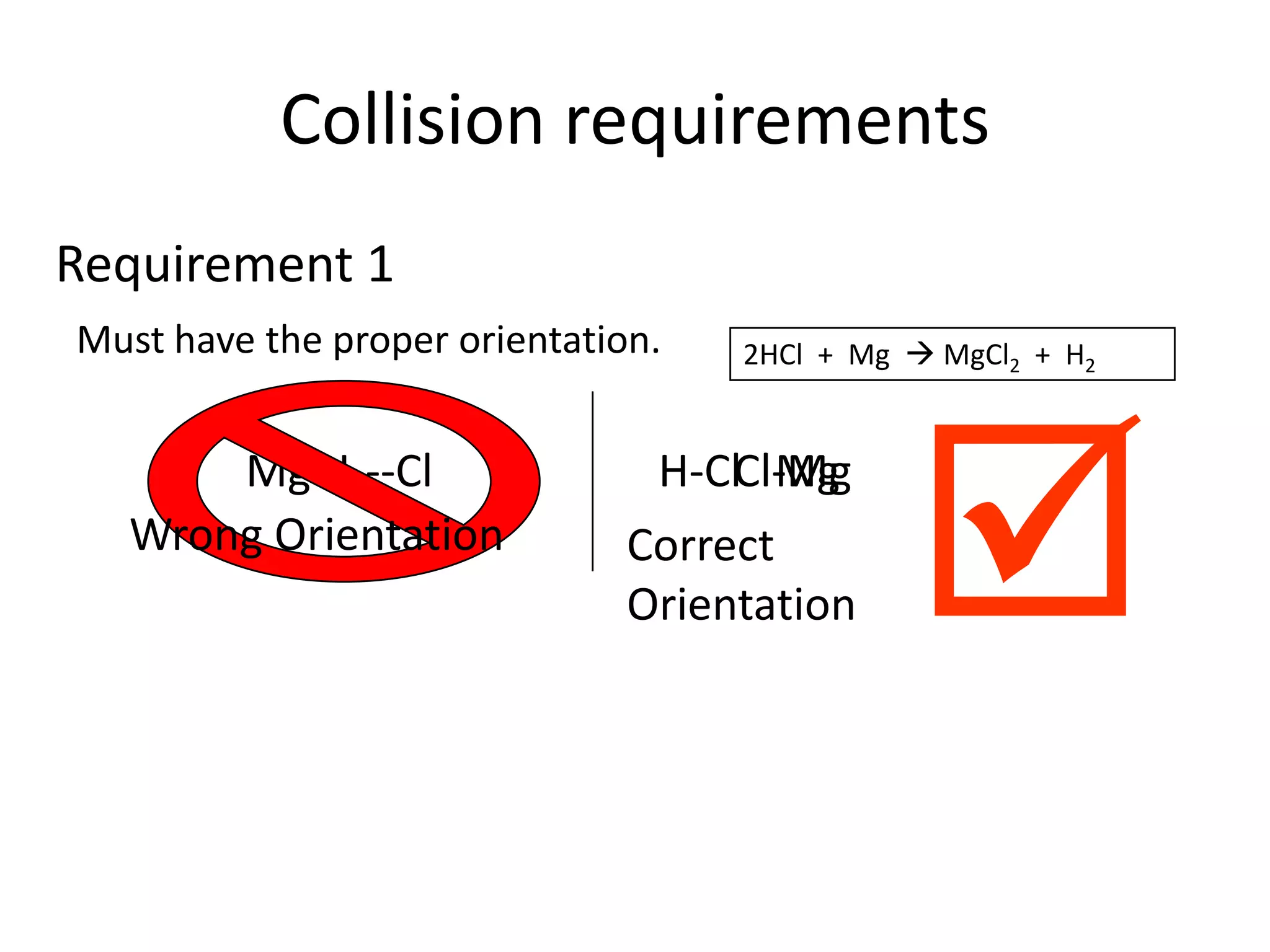
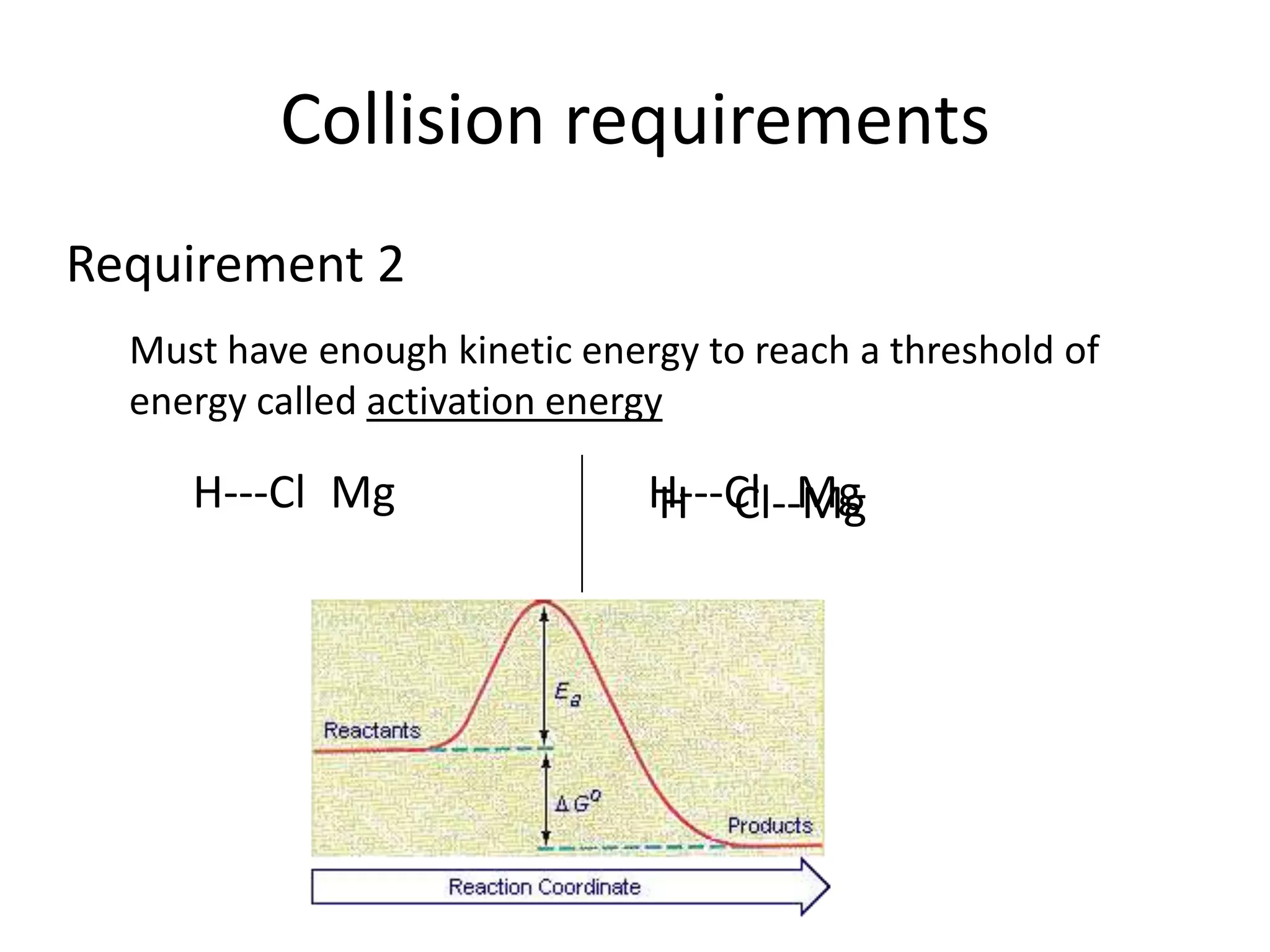
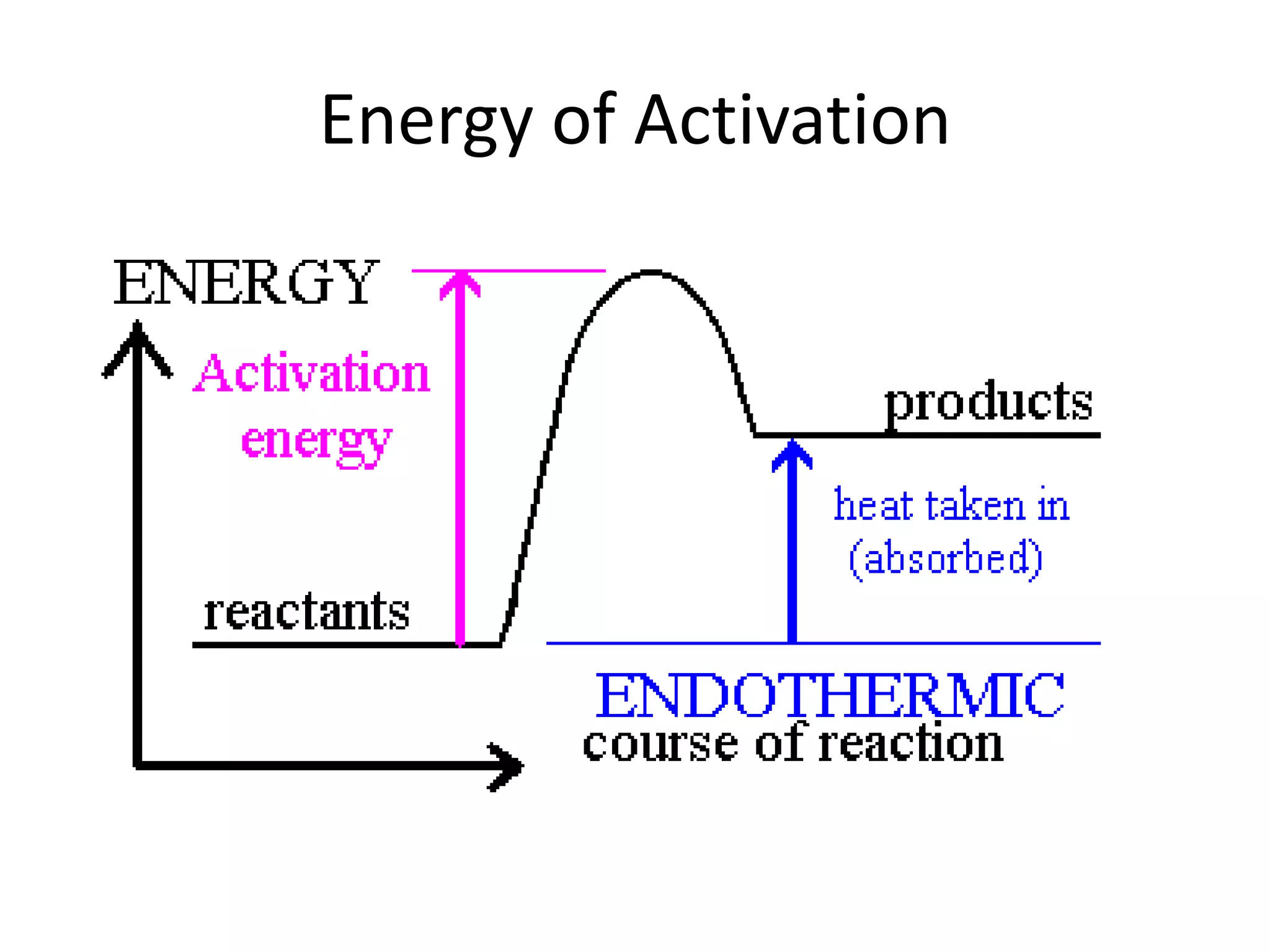
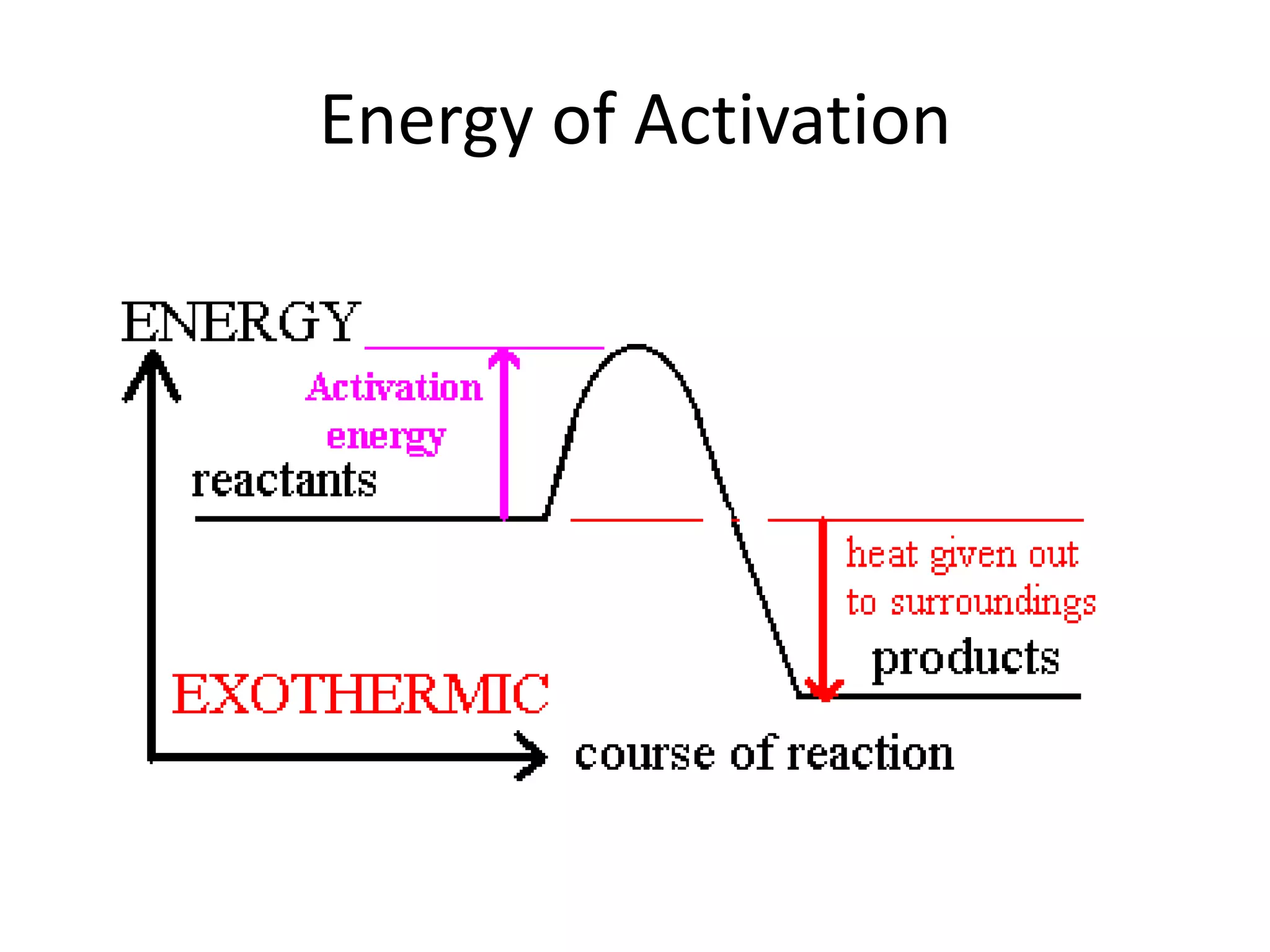

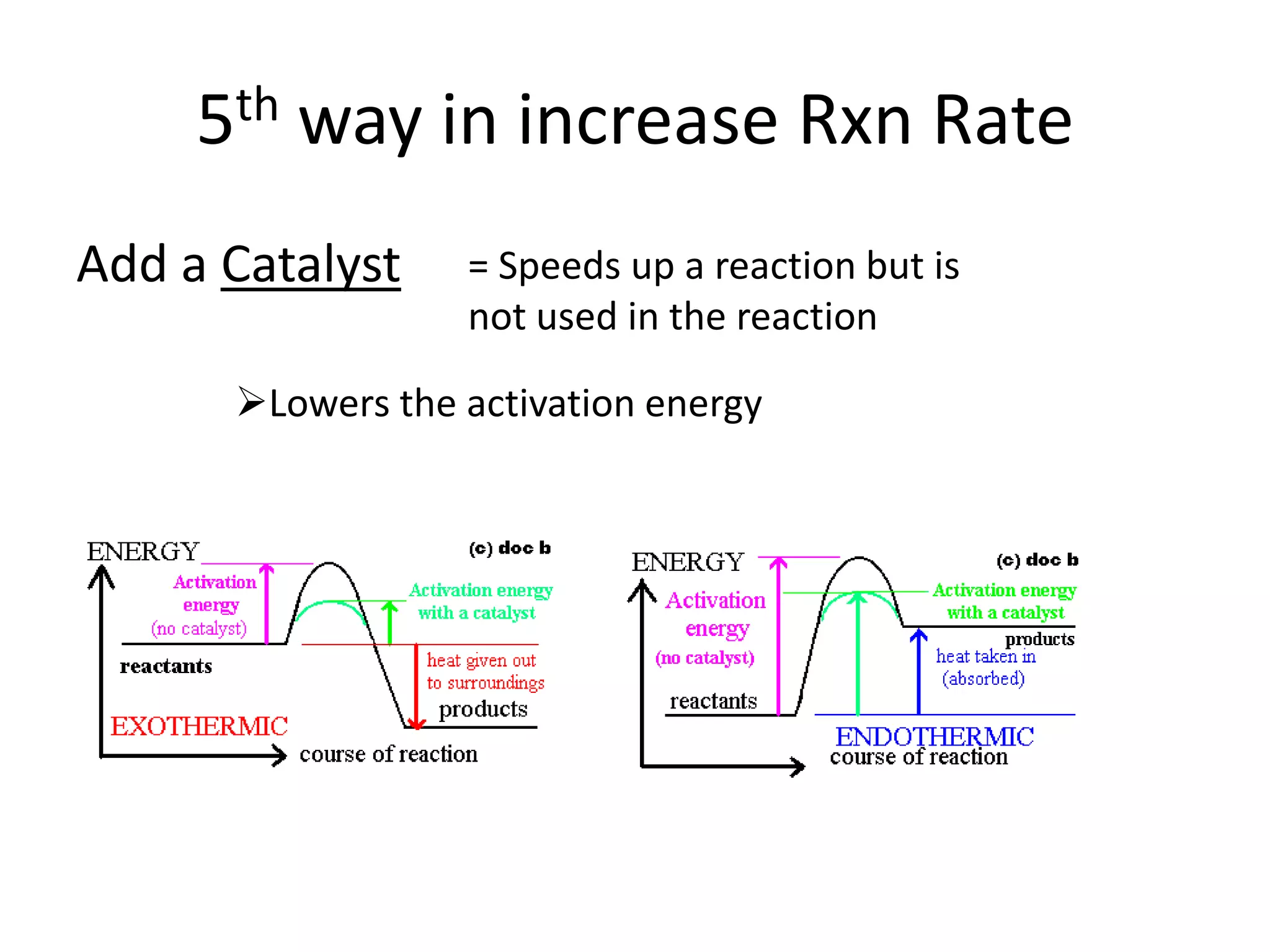
Collisions between reactants are necessary for chemical reactions to occur. The reactants must collide with the proper orientation and with sufficient kinetic energy to overcome the activation energy barrier. Adding a catalyst can also increase the reaction rate by lowering the activation energy needed for collisions between reactants to be productive.






