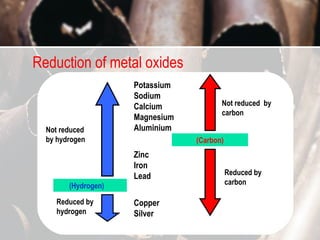
This chapter discusses the physical and chemical properties of metals. Metals are usually hard, shiny, malleable and ductile. They are good conductors of heat and electricity. Chemically, metals form positive ions and react with acids, oxygen, water and steam to form salts and release hydrogen gas. The reactivity of metals can be predicted based on their reactivity series, with more reactive metals displacing less reactive ones from their compounds. Alloys are stronger than pure metals due to disrupted atomic layers.