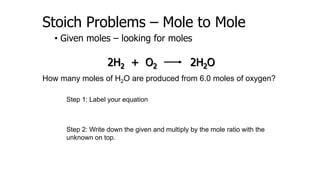
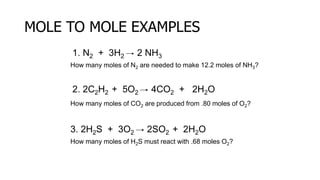
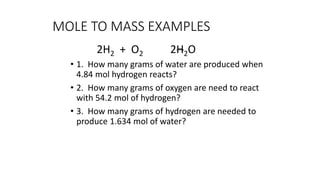
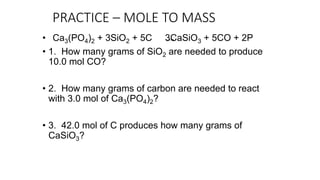
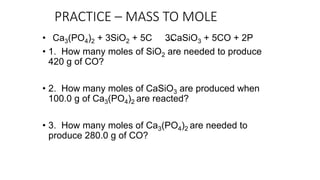
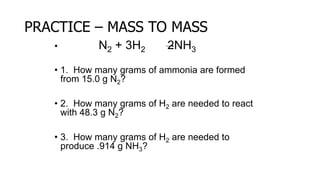
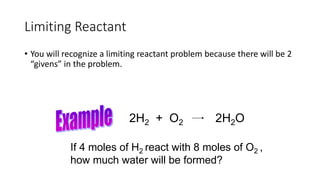
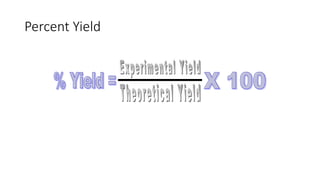
The document discusses stoichiometric relationships and reacting masses and volumes. It provides examples of mole to mole, mole to mass, mass to mole, and mass to mass stoichiometry problems. It also covers limiting reactants, theoretical yield, experimental yield, and calculating percent yield. The key ideas are that mole ratios can be used to calculate reacting quantities, the limiting reactant determines the theoretical yield, the experimental yield may differ from the theoretical yield, and percent yield compares the experimental to the theoretical yield.