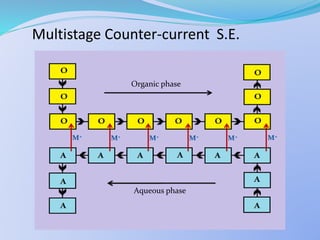
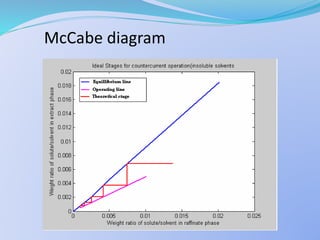
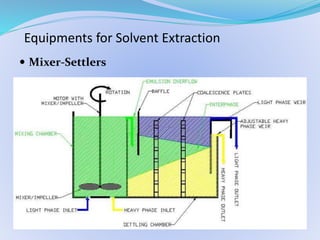
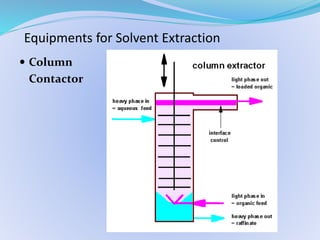
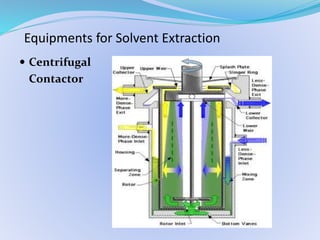
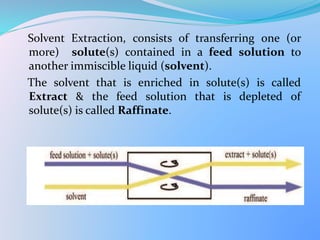
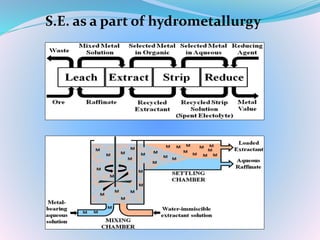
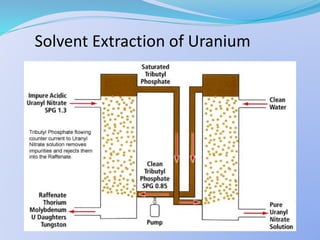
Solvent extraction, also known as liquid-liquid extraction, is a method used to separate compounds based on their relative solubilities in two different immiscible liquids, usually water and an organic solvent. It involves transferring solutes from a feed solution to another immiscible liquid called the extract. Solvent extraction was first developed in analytical chemistry and is now widely used in hydrometallurgy and other industries to separate and extract various metals, rare earth elements, and other compounds. Key aspects of solvent extraction processes include the selection of appropriate organic solvents and diluents, extraction equipment like mixer-settlers or columns, and multi-stage counter-current contacting to efficiently separate solutes.









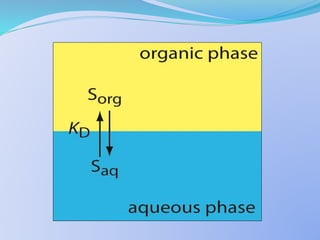
![Extraction Equilibria
For a chelating reagent, HR, the extraction reaction for
a metal ion Mn+ can be represented as:
Maq
n+ + n HRorg MRn org + H+
aq
The Extraction Constant(Kex) is given by:
Kex =
[MRn]org [H+]n
aq
[Mn+]aq [HR]n
org](https://image.slidesharecdn.com/solventextraction-150817130936-lva1-app6892/85/Solvent-Extraction-16-320.jpg)
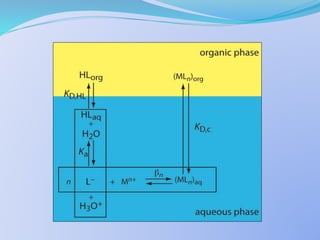
![The extraction constant can also be expressed as:
Kex =
KD .[H+]n
aq
[HR]n
org
Where,
KD =
[MRn]org
[Mn+]aq](https://image.slidesharecdn.com/solventextraction-150817130936-lva1-app6892/85/Solvent-Extraction-17-320.jpg)
![The extraction constant can also be expressed as:
Kex =
log KD = log Kex + n pH + n log [HR]org
KD .[H+]n
aq
[HR]n
org
Where,
KD =
[MRn]org
[Mn+]aq](https://image.slidesharecdn.com/solventextraction-150817130936-lva1-app6892/85/Solvent-Extraction-18-320.jpg)
![Extraction Coefficient
It indicates how much of the metal will move from
the aqueous phase to the organic phase in a single
contact.
Effective extraction coefficient (EF) can be expressed as:
EF =
[Mn+]org (Vol.)org
[Mn+]aq (Vol.)aq](https://image.slidesharecdn.com/solventextraction-150817130936-lva1-app6892/85/Solvent-Extraction-19-320.jpg)
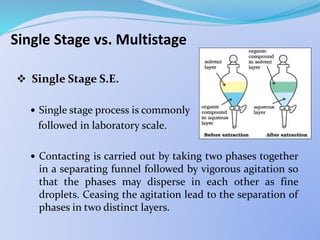
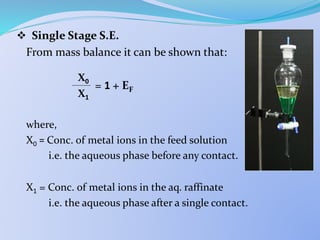
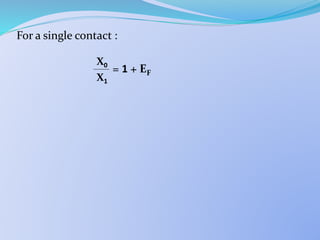
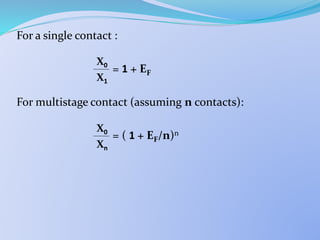
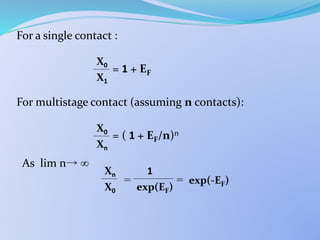
![ Single Stage S.E.
From mass balance it can be shown that:
= 1 +
where,
X0 = Conc. of metal ions in the feed solution
i.e. the aqueous phase before any contact.
X1 = Conc. of metal ions in the aq. raffinate
i.e. the aqueous phase after a single contact.
X1
X0
[Mn+]org (Vol.)org
[Mn+]aq (Vol.)aq](https://image.slidesharecdn.com/solventextraction-150817130936-lva1-app6892/85/Solvent-Extraction-21-320.jpg)