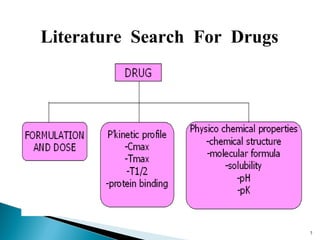
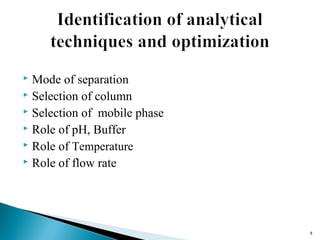
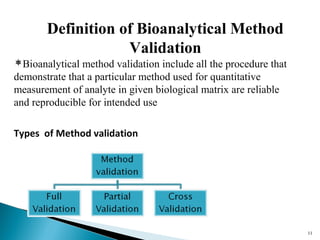
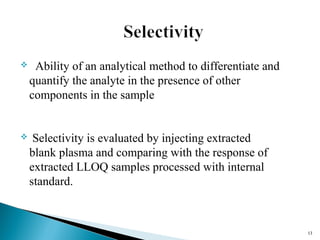
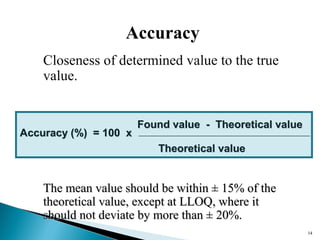
The document discusses the development and validation of an LC-MS/MS bioanalytical method for the determination of a drug in human plasma. It describes the objectives, which are to develop a specific, reliable and cost-effective method. It outlines the key steps in method development including literature search, reference standard preparation, extraction procedure selection, and optimization of separation and detection. The validation parameters that will be evaluated are also summarized, including specificity, accuracy, precision, recovery, matrix effects and stability.