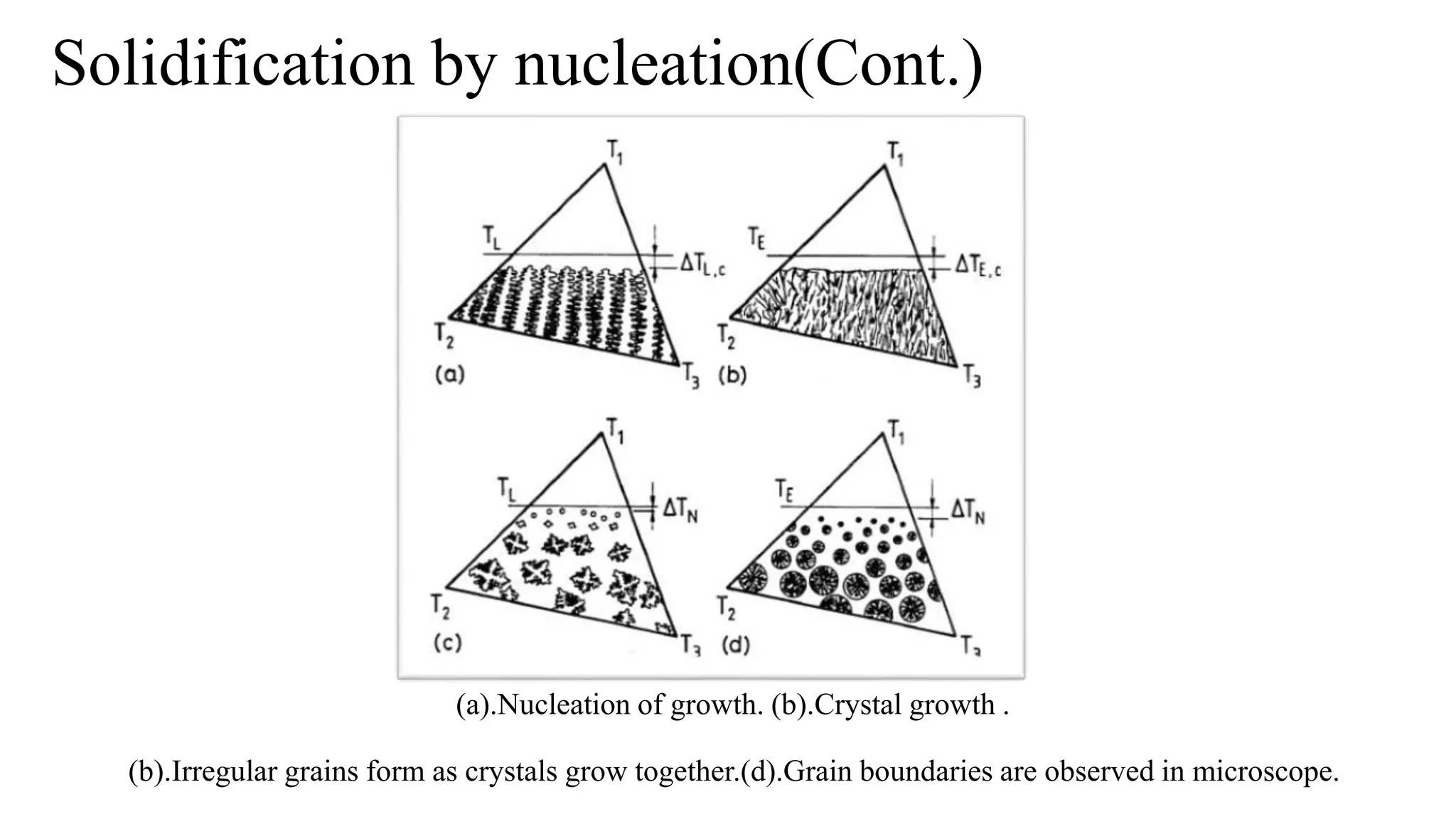
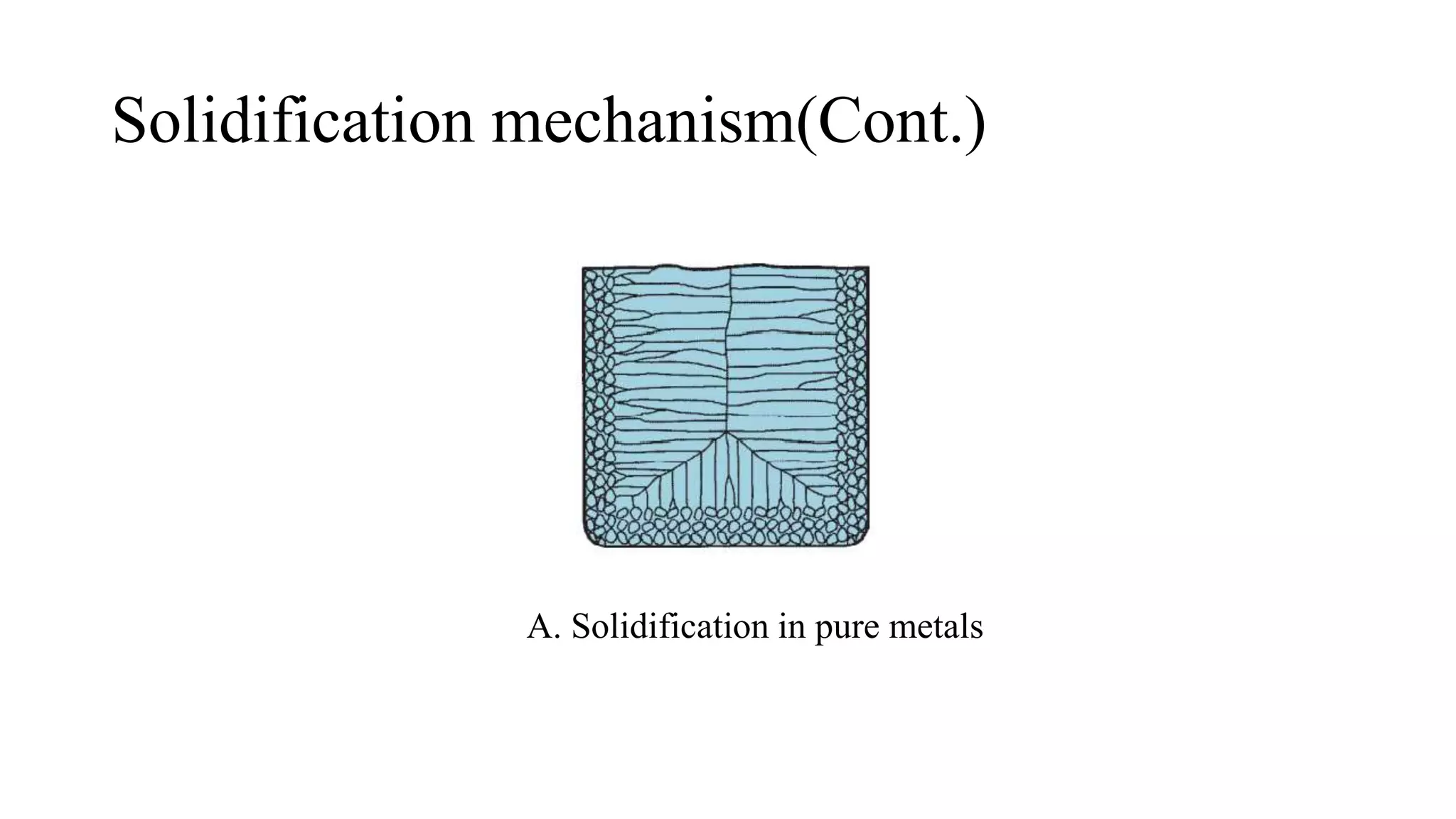
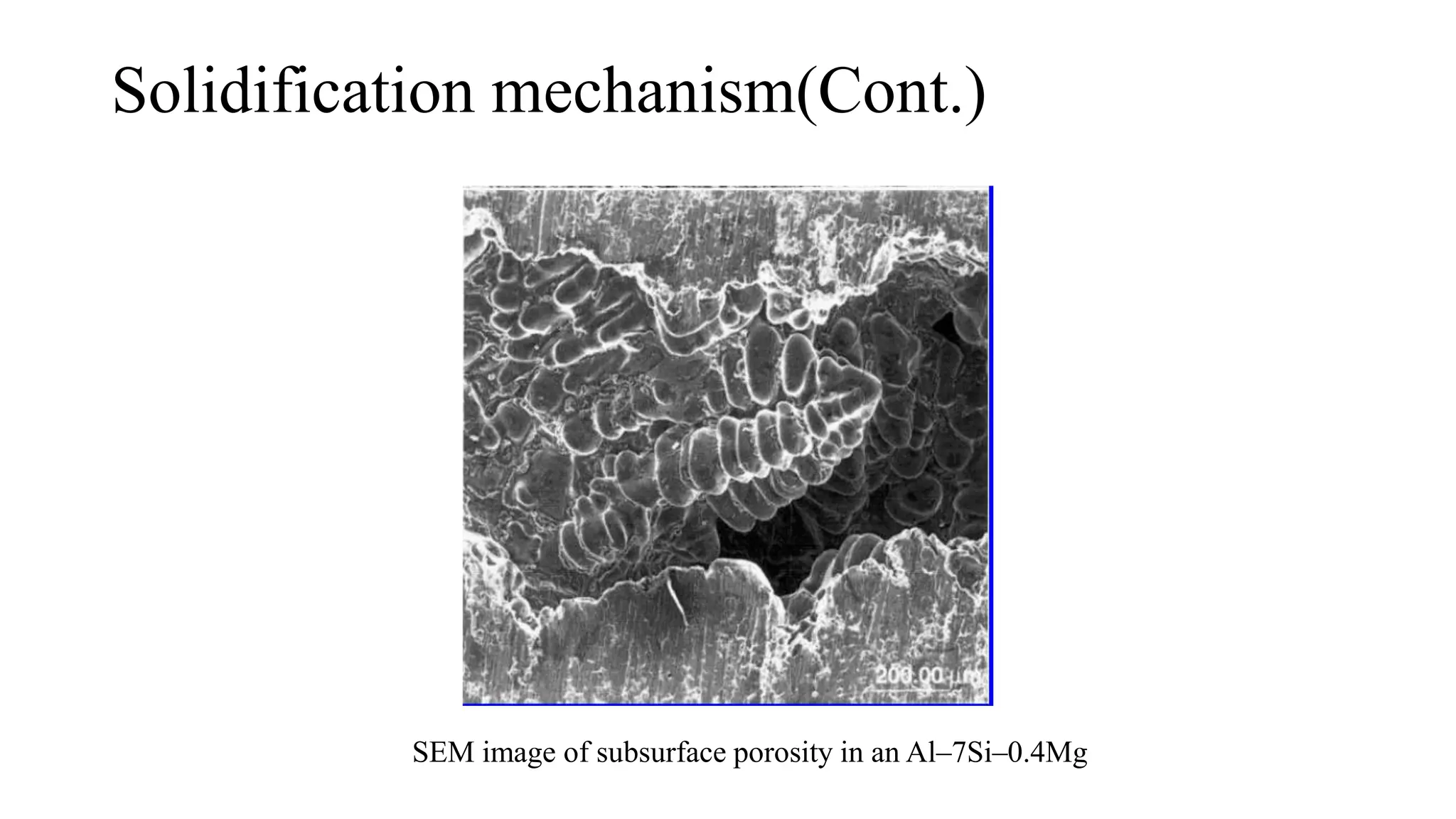
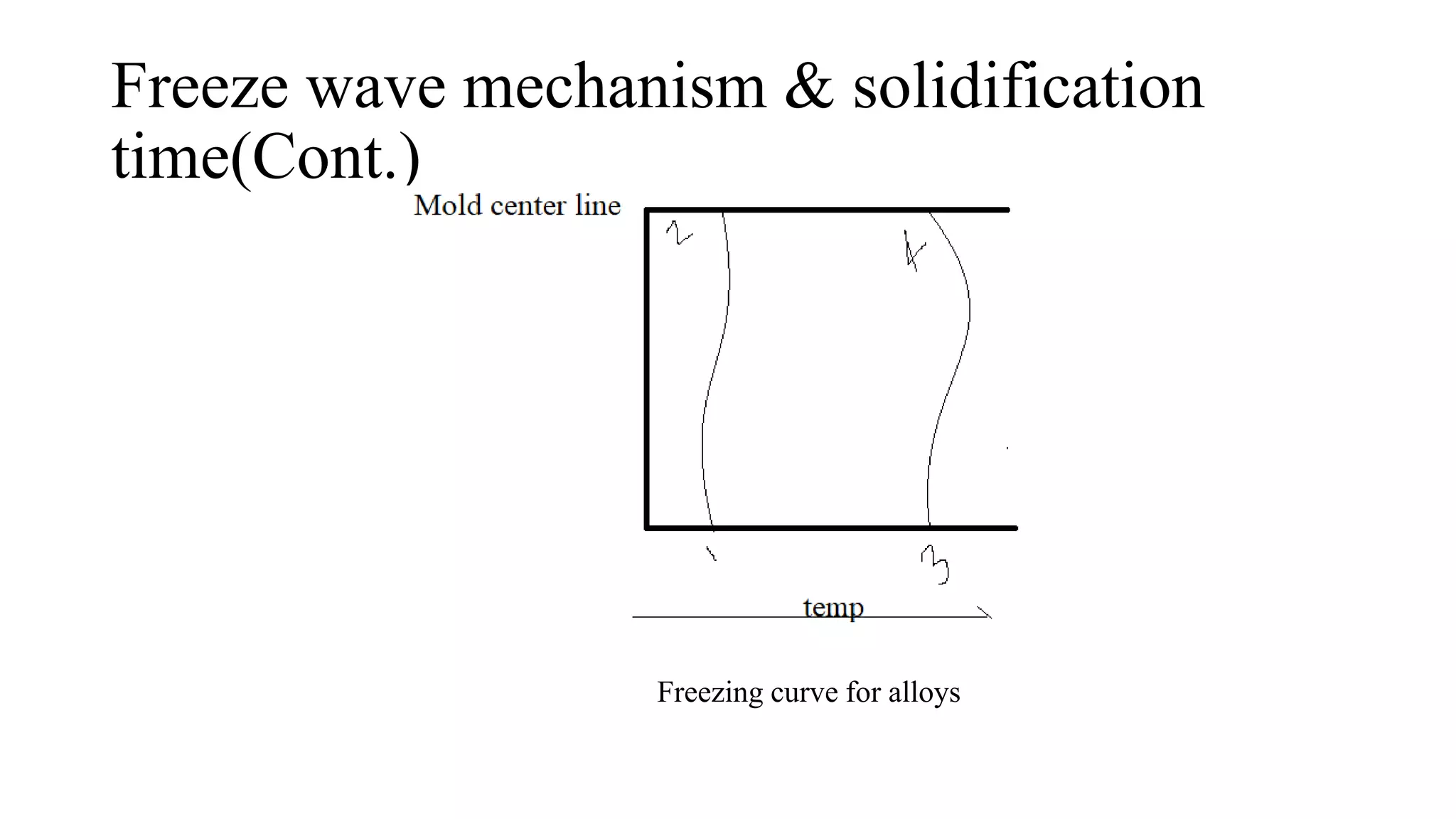
The document discusses the solidification process of metals and plastics during casting, detailing steps such as nucleation, solidification mechanisms, and defects that may occur. It emphasizes the significance of nucleation in crystal formation and various mechanisms that influence solidification times, specifically in alloys, as well as the application of Chvorinov's rule. Additionally, it covers common solidification defects like shrinkage and gas porosity, supported by references for further reading.