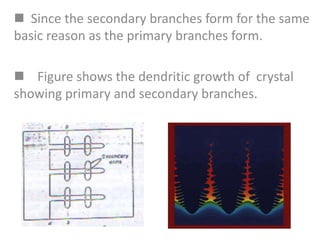
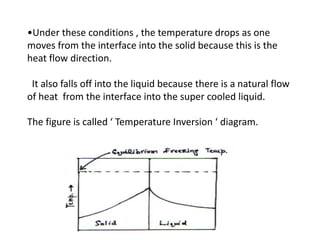
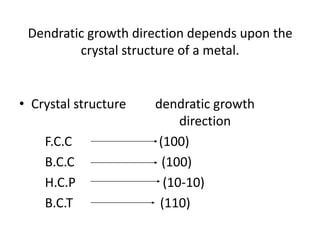
- Dendritic crystal growth occurs when a liquid-solid interface moves into a supercooled liquid. Heat is removed from the interface into both the solid and liquid.
- Undercooling of the liquid allows the formation of spikes at the interface that grow faster than the surrounding interface. This leads to the formation of branched, tree-like dendritic structures.
- Secondary and tertiary branches can form from primary branches/spikes. The branching occurs due to temperature gradients that arise from the release of heat at the interface.

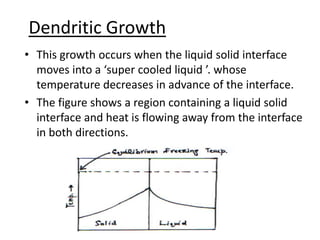


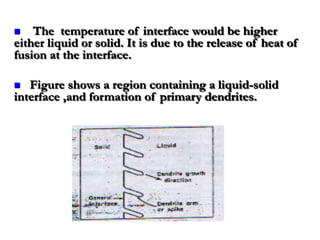


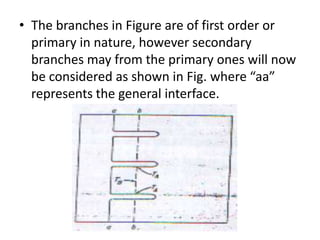
![• Once the spikes have formed growth of
general interface will be slow because here
the supper cooling is small.
• At section “bb” on the other hand the average
temperature of the liquid is lower than at “aa”
due to the release of latent heat of fusion at
the spikes, the temperature TA (at the spikes )
will be higher as compared to TB (between the
spikes ). [ TA >TB ]](https://image.slidesharecdn.com/dendraticgrowth-130121012310-phpapp01/85/Dendratic-growth-13-320.jpg)