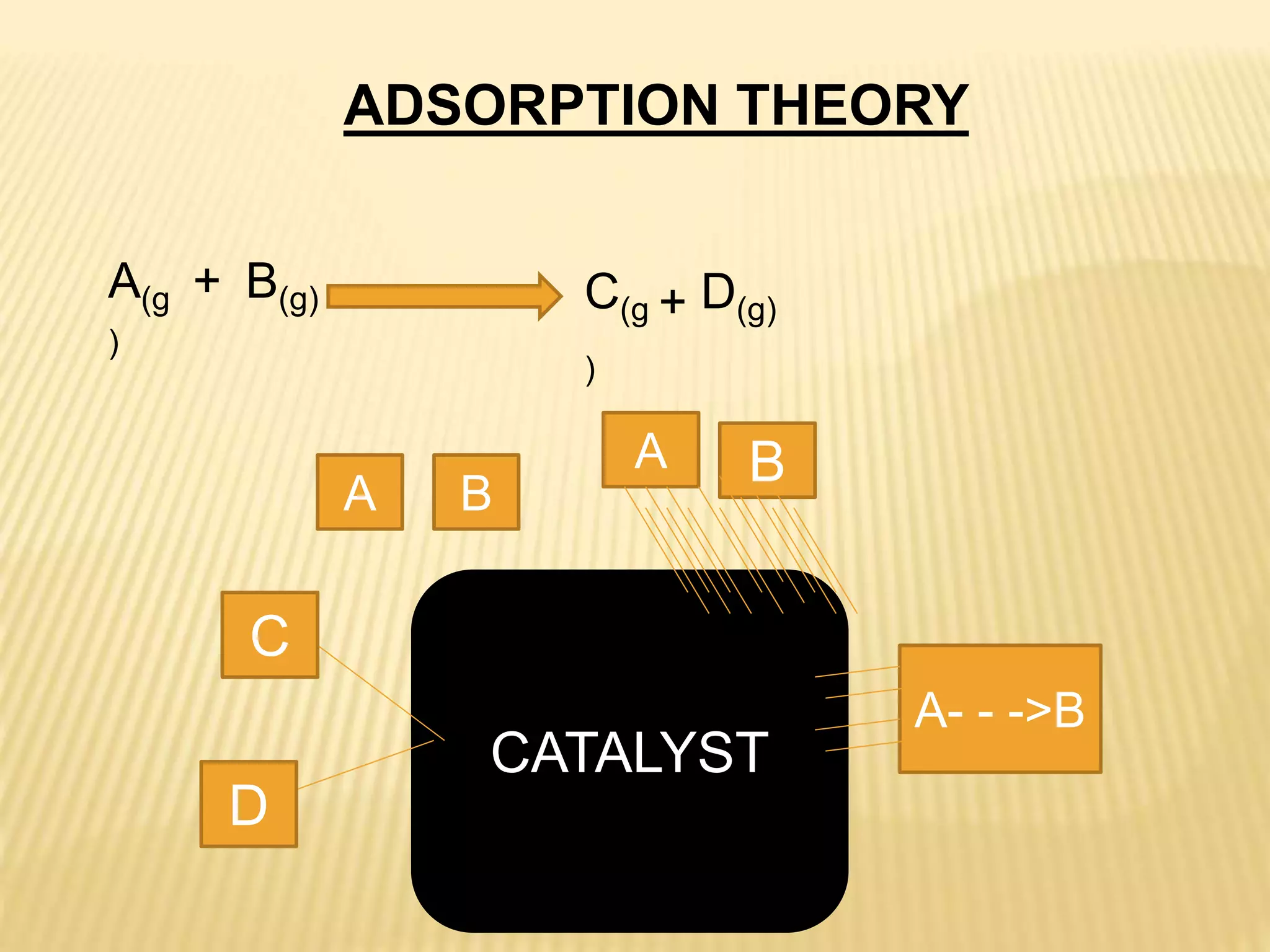
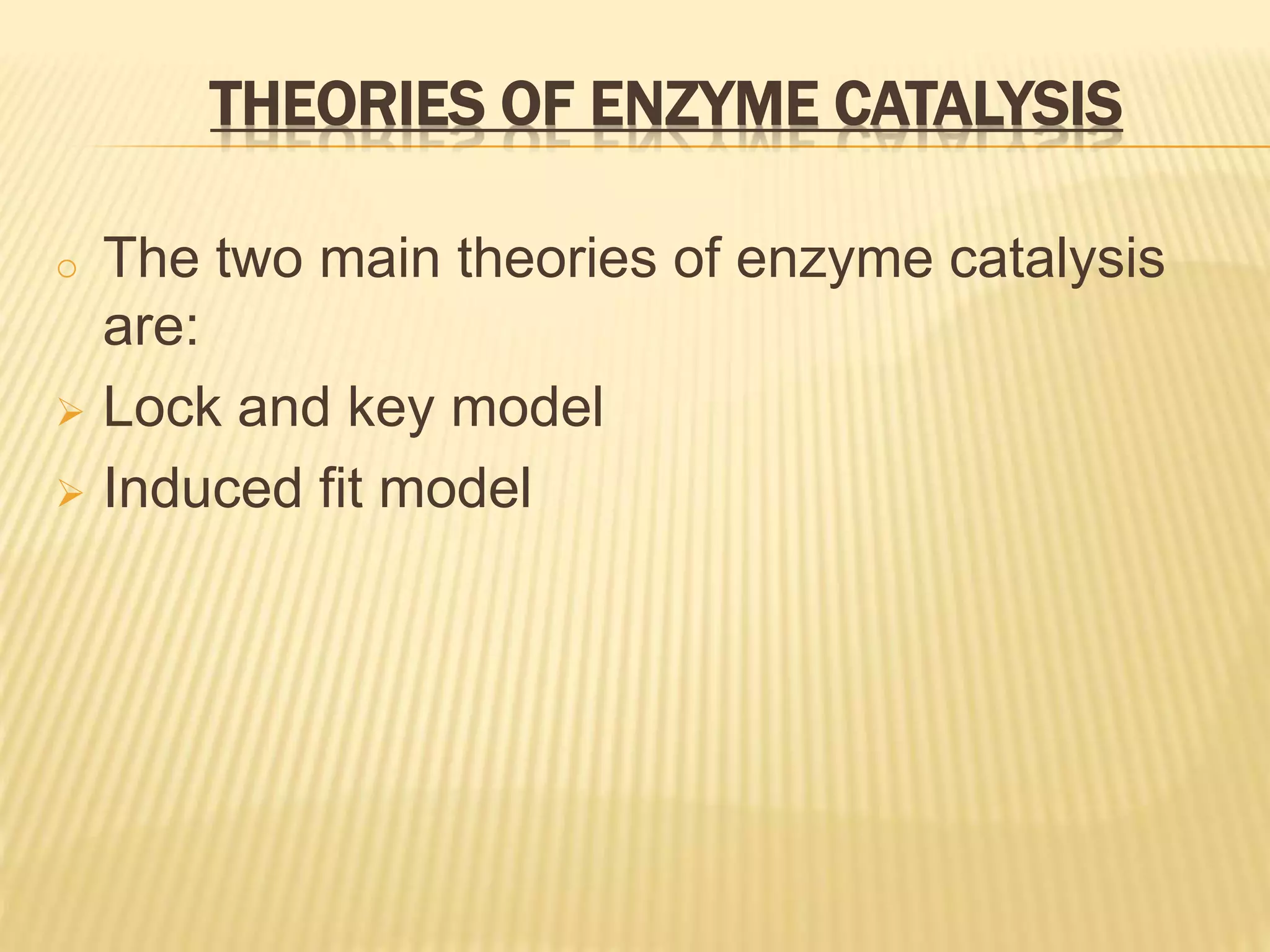
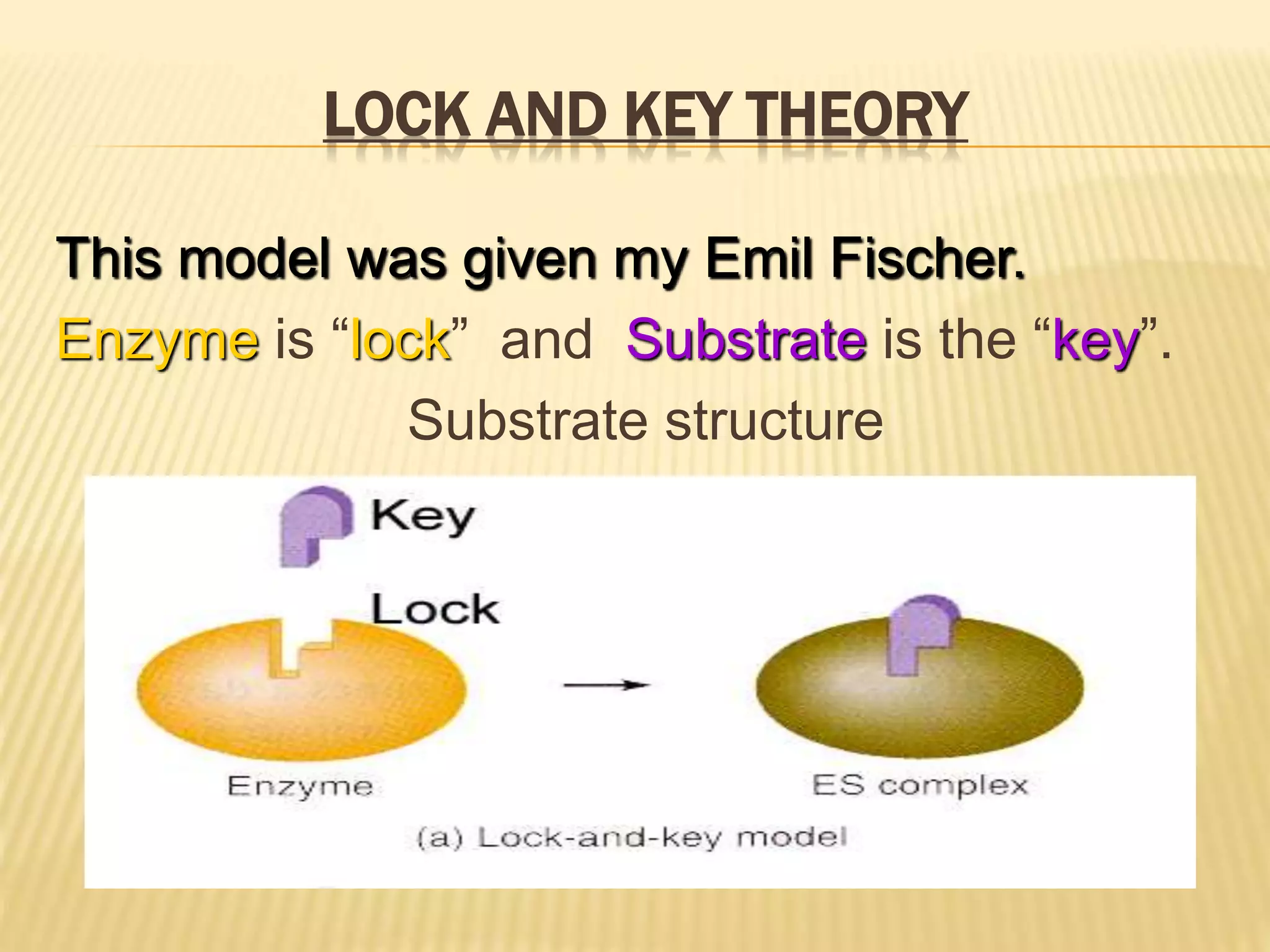
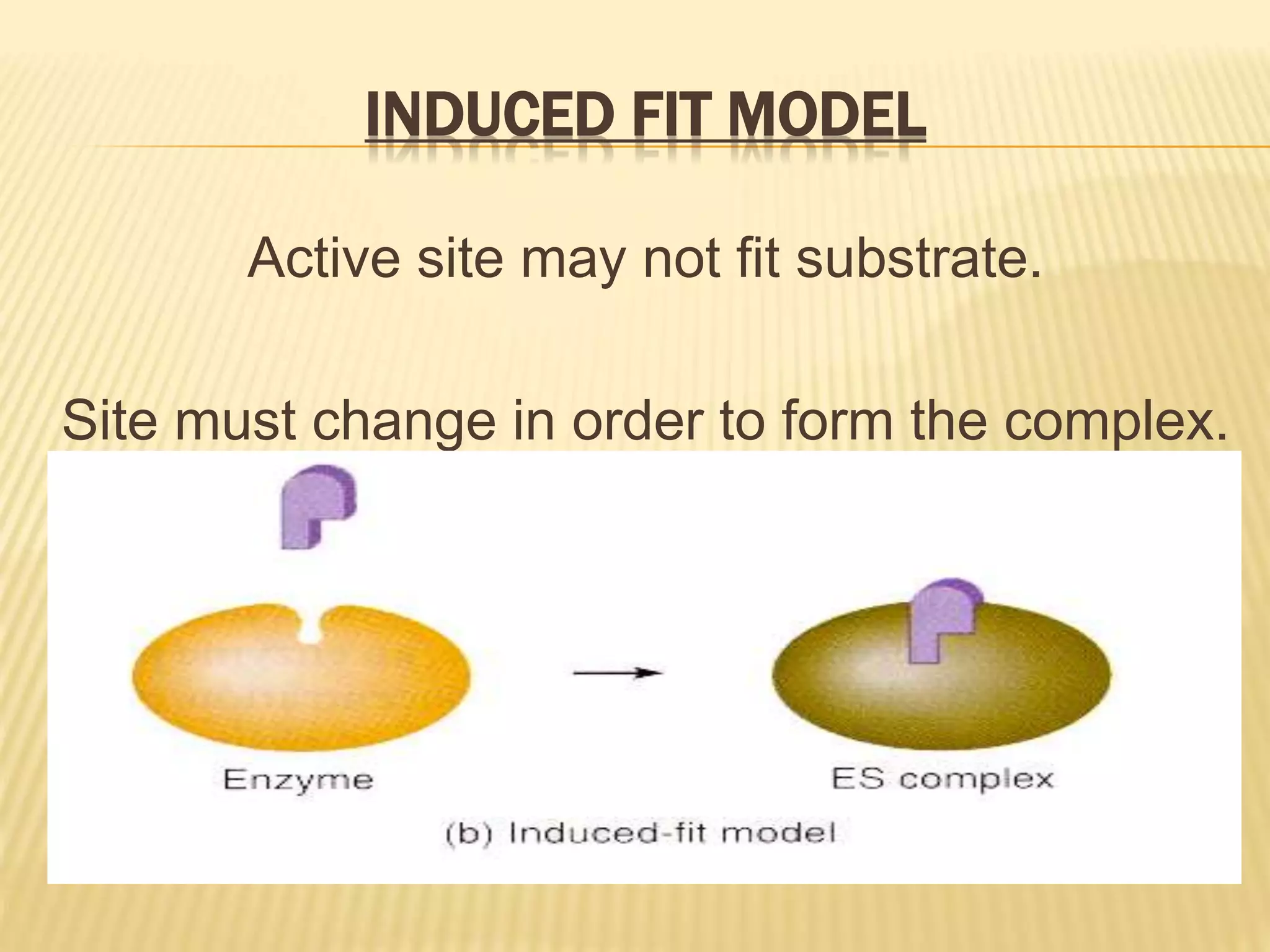
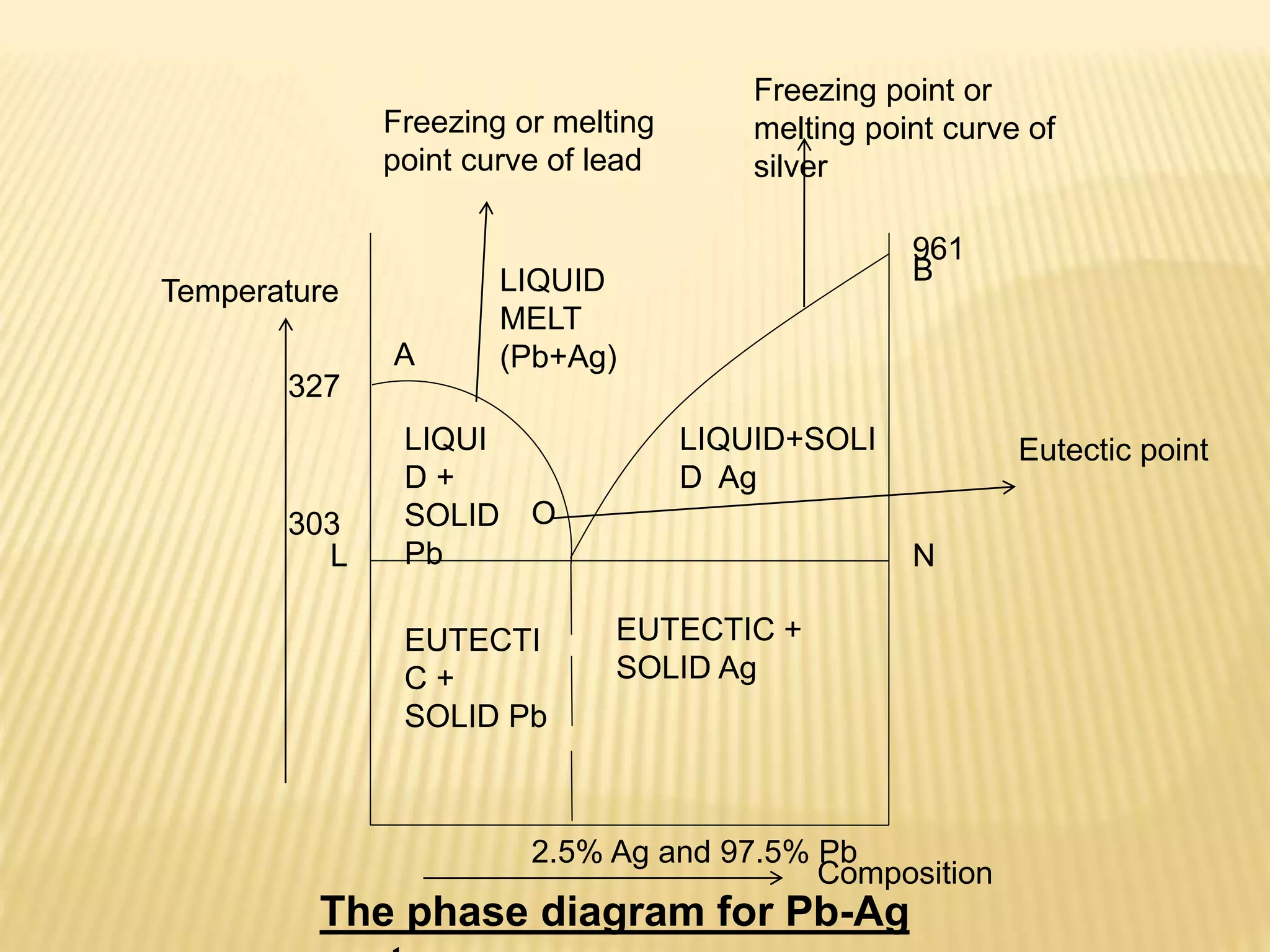
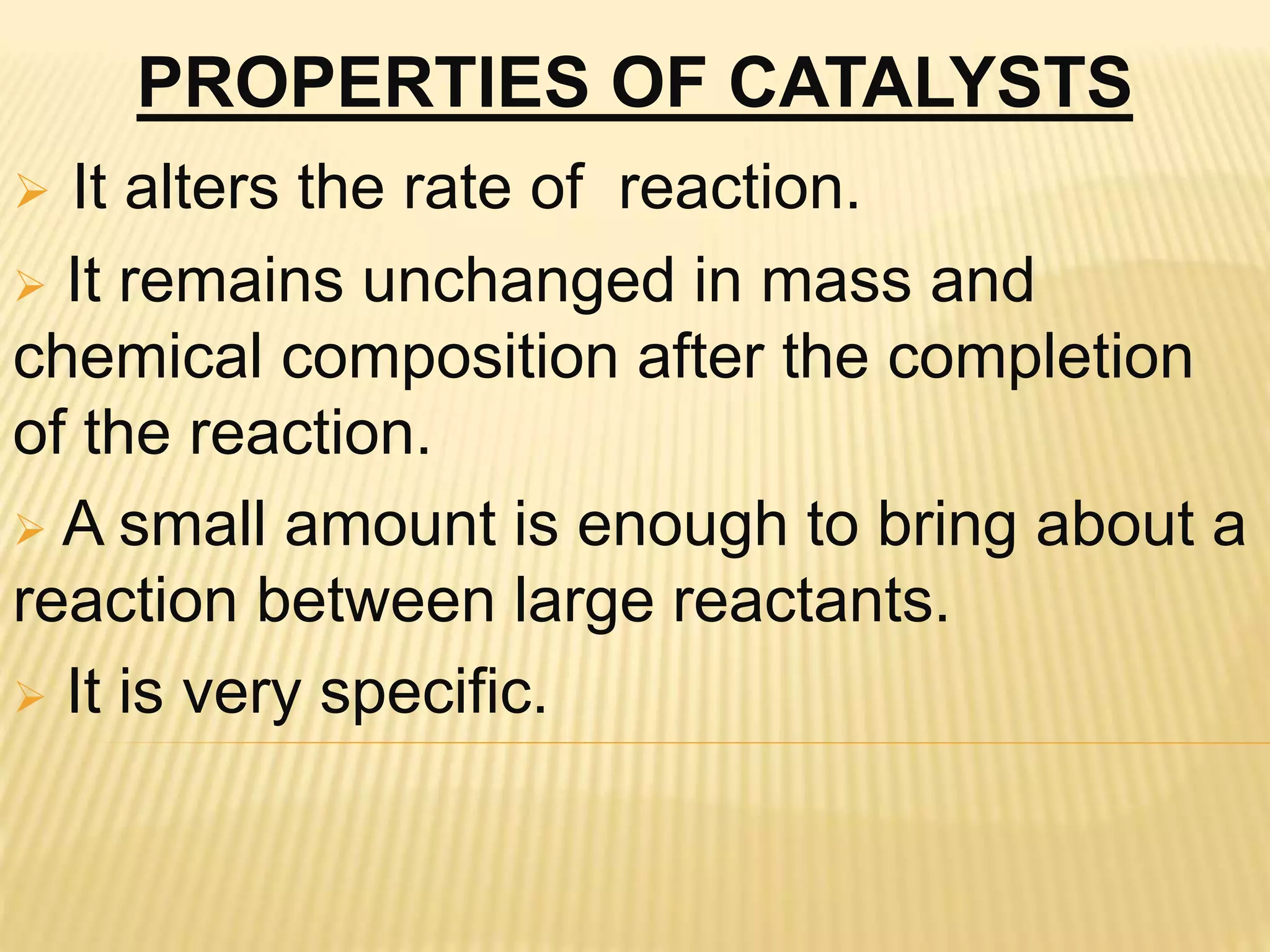
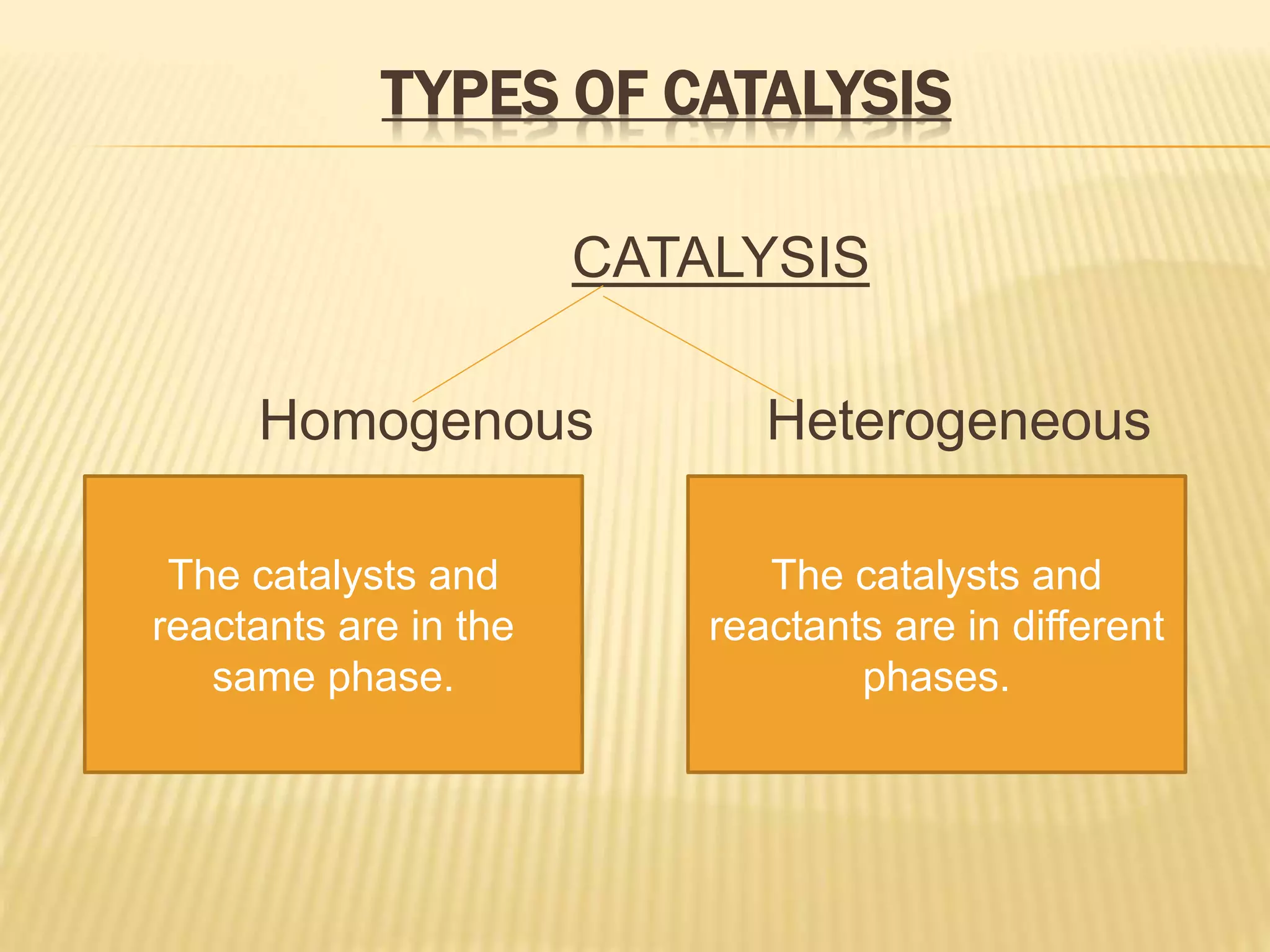
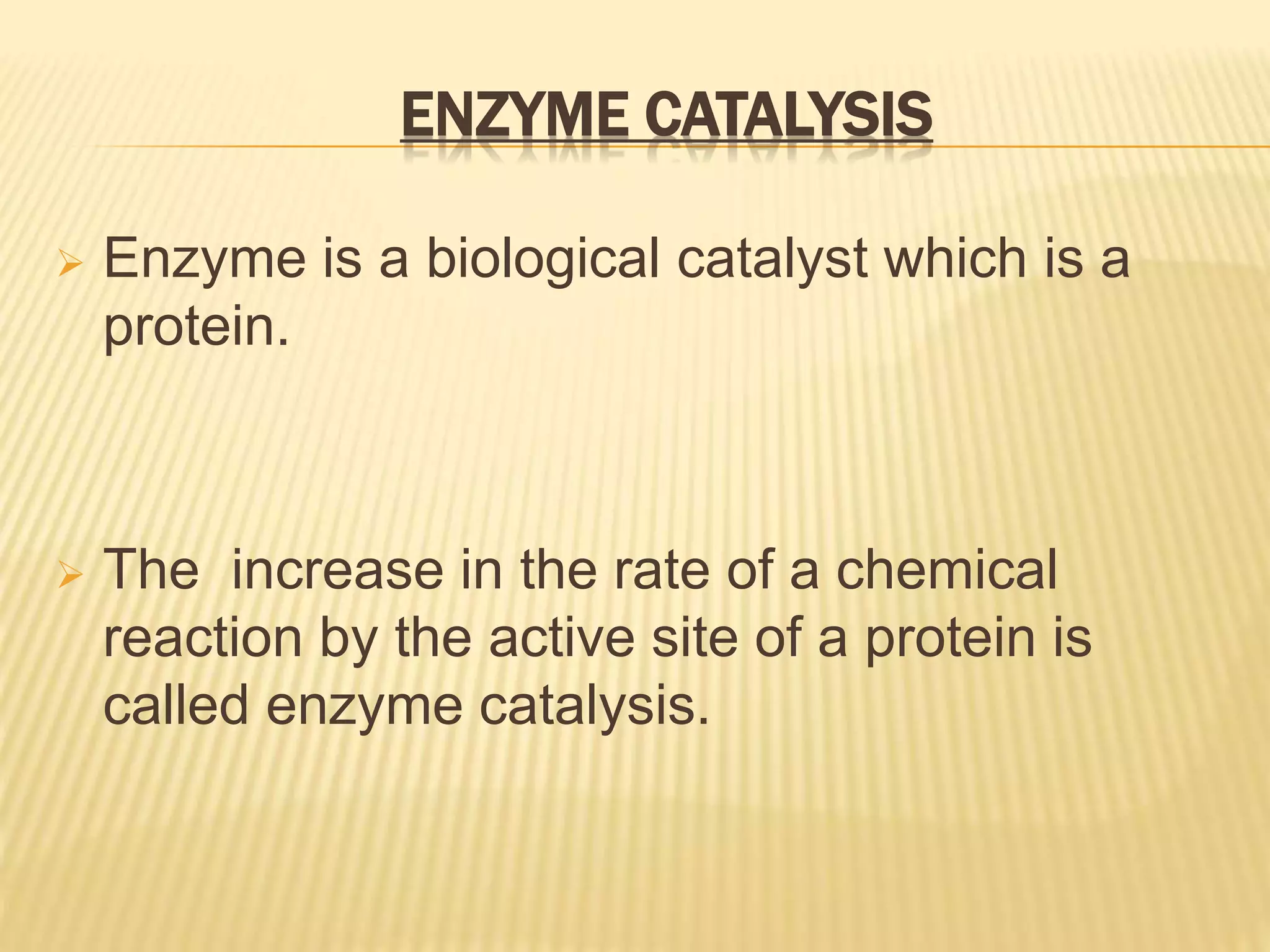
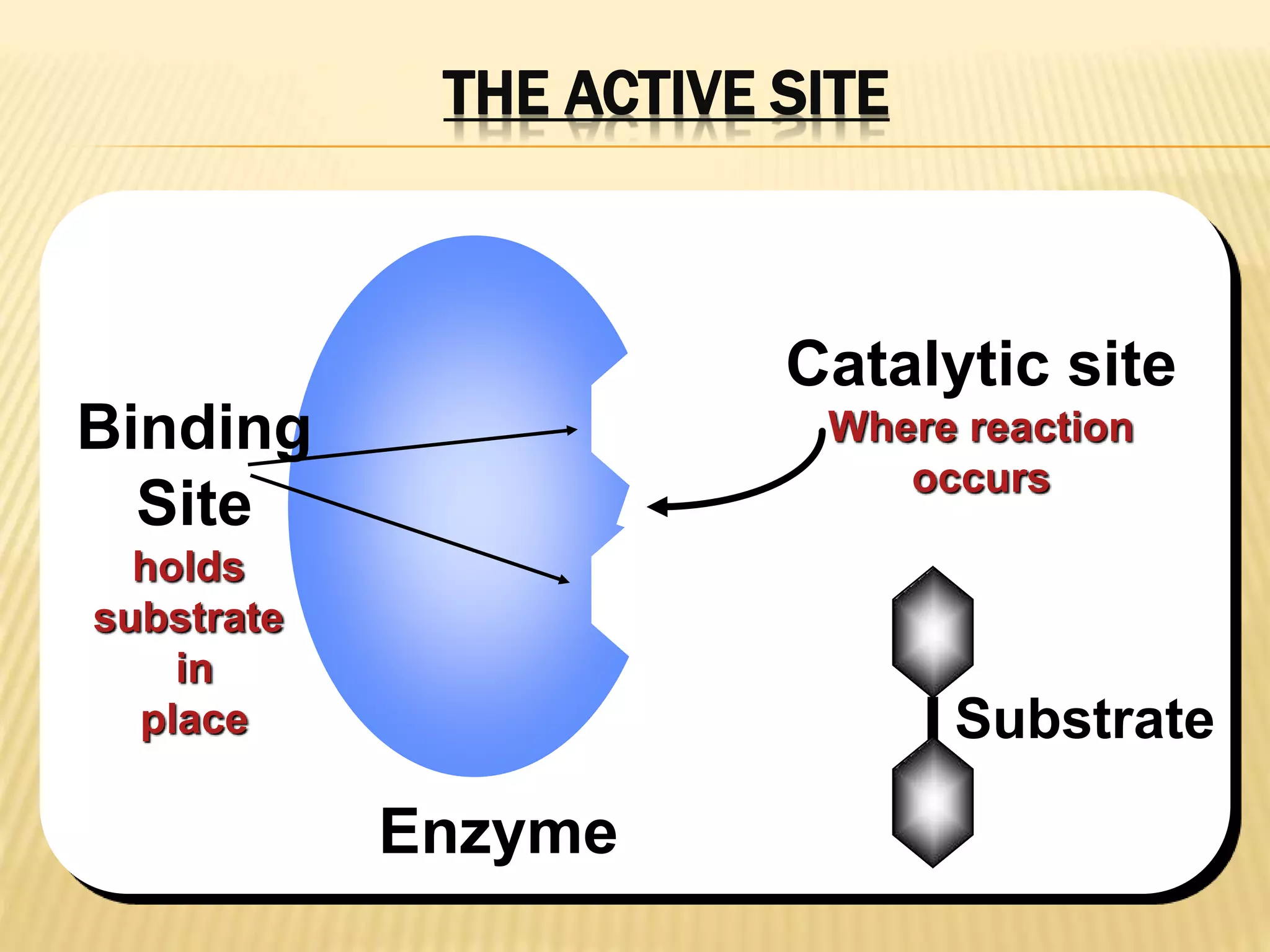
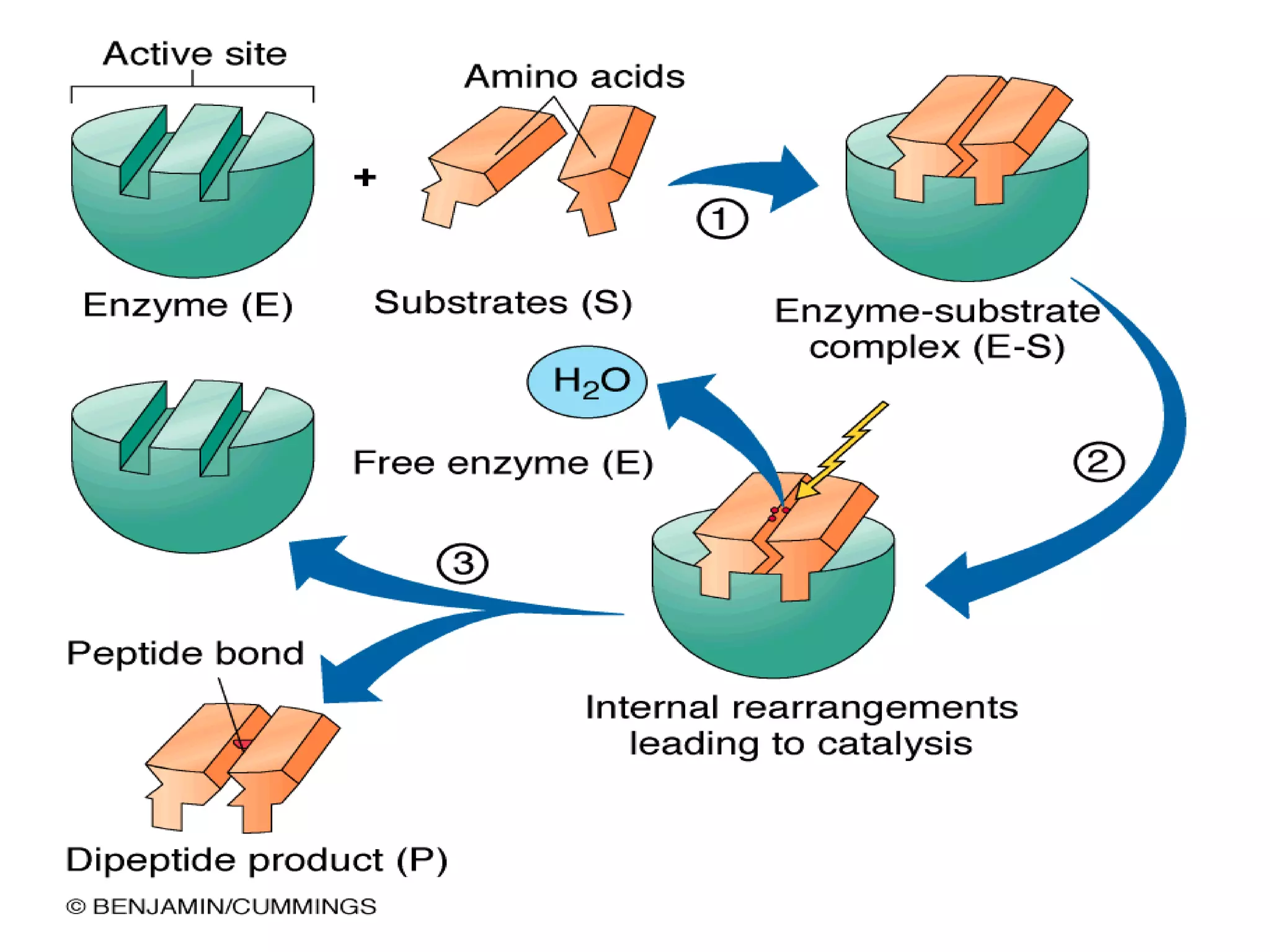
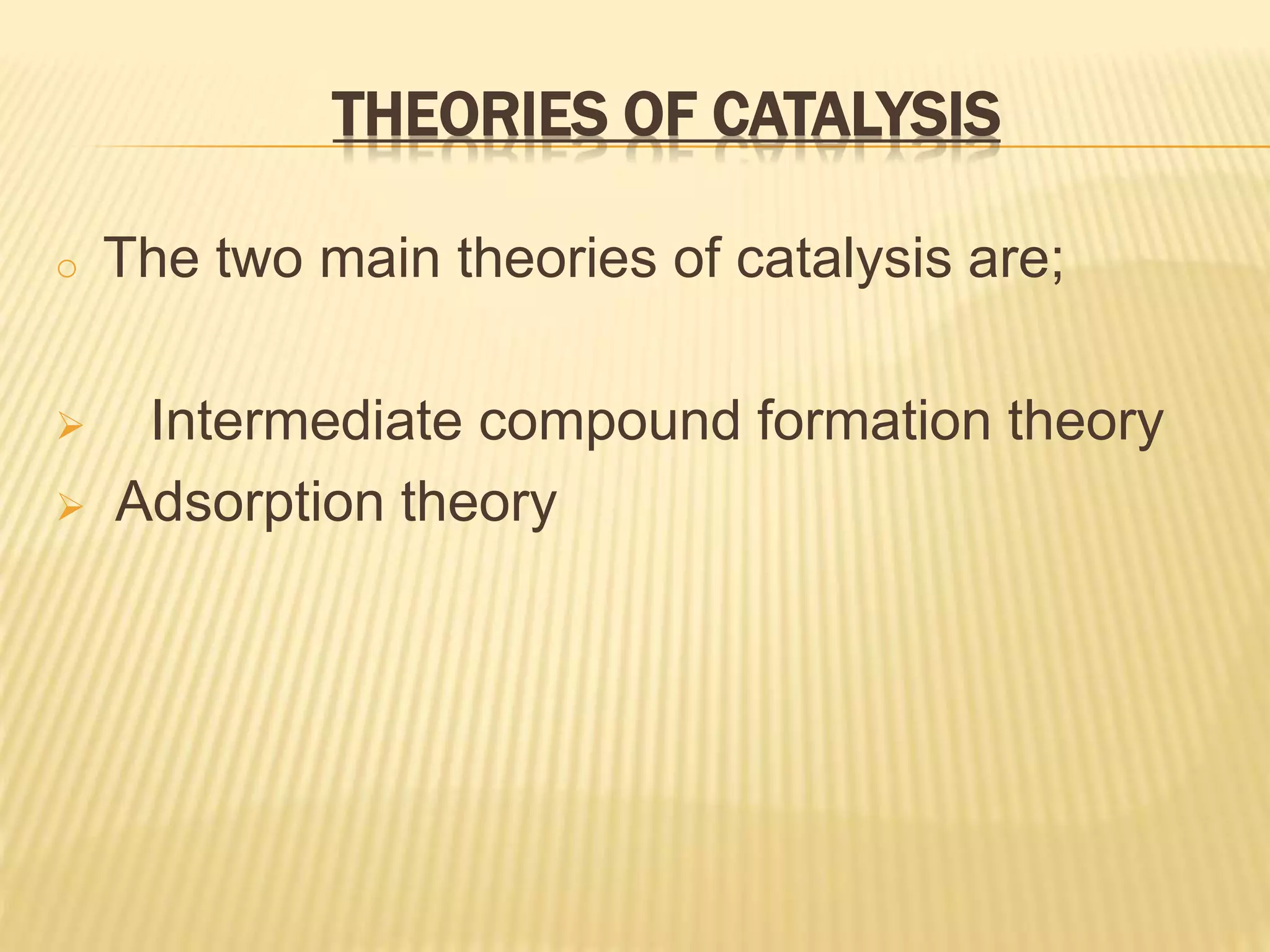
This document summarizes information about simple eutectic systems and enzyme catalysis. It first discusses the lead-silver eutectic system, noting that lead and silver are completely miscible in liquid state but immiscible in solid state, forming a simple eutectic system. The phase diagram shows the eutectic point occurs at 2.5% silver and 97.5% lead with a temperature of 327°C. It then discusses enzyme catalysis, noting enzymes are protein catalysts that increase reaction rates via an active site. The two main theories of catalysis are intermediate compound formation and adsorption, while the two main theories of enzyme catalysis are the lock-and-key and induced fit








![INTERMEDIATE COMPOUND FORMATION
THEORY
A + [CATALYST] [INTERMEDIATE]
[INTERMEDIATE] + B PRODUCT +
CATALYST
2SO2 + O2
[NO]
2SO3
Example:
2NO + O2 2NO2
NO2 + SO2 SO3 + NO](https://image.slidesharecdn.com/simpleeutecticsystempb-ag-160128124624/75/Simple-eutectic-system-pb-ag-19-2048.jpg)