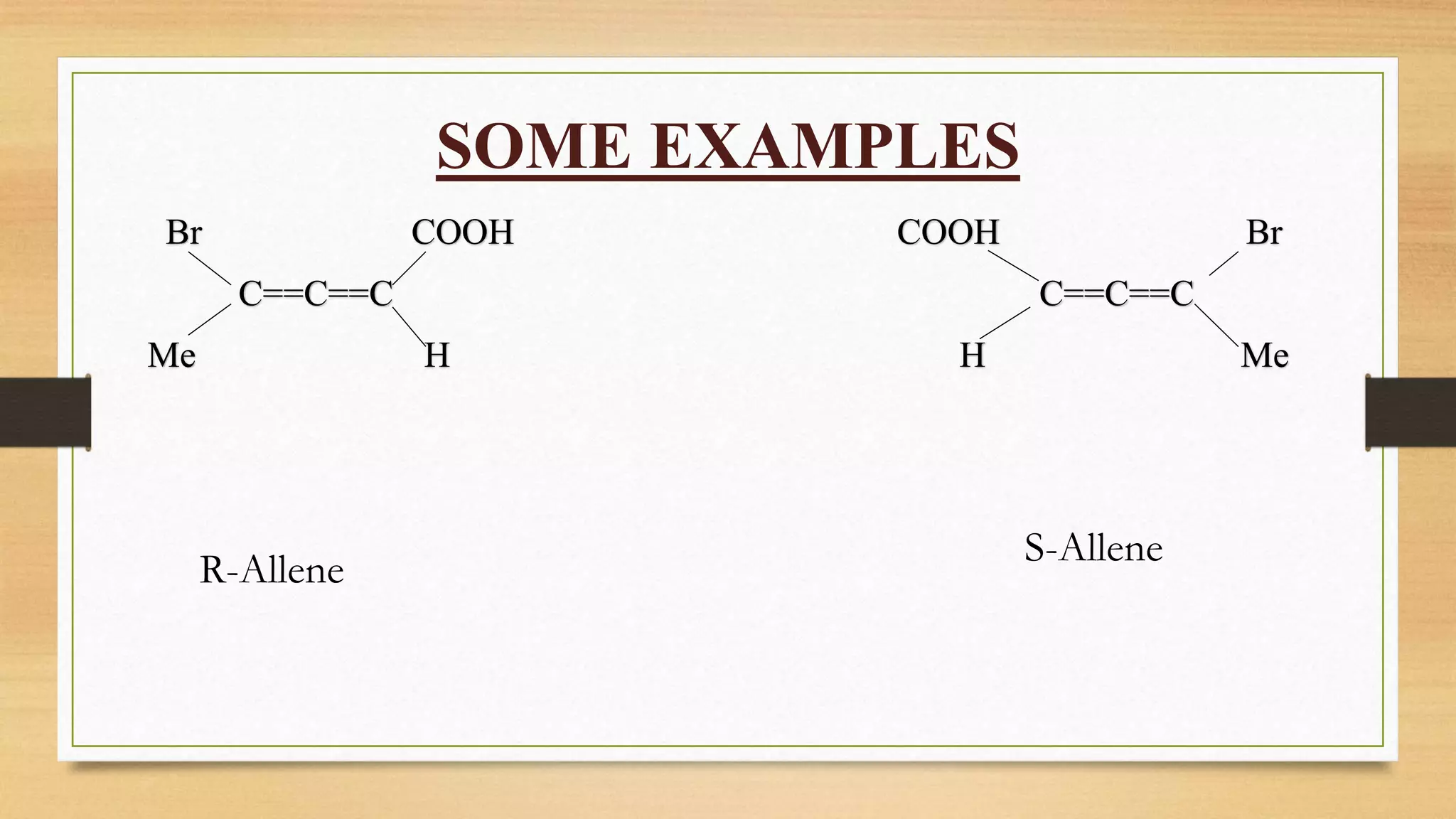
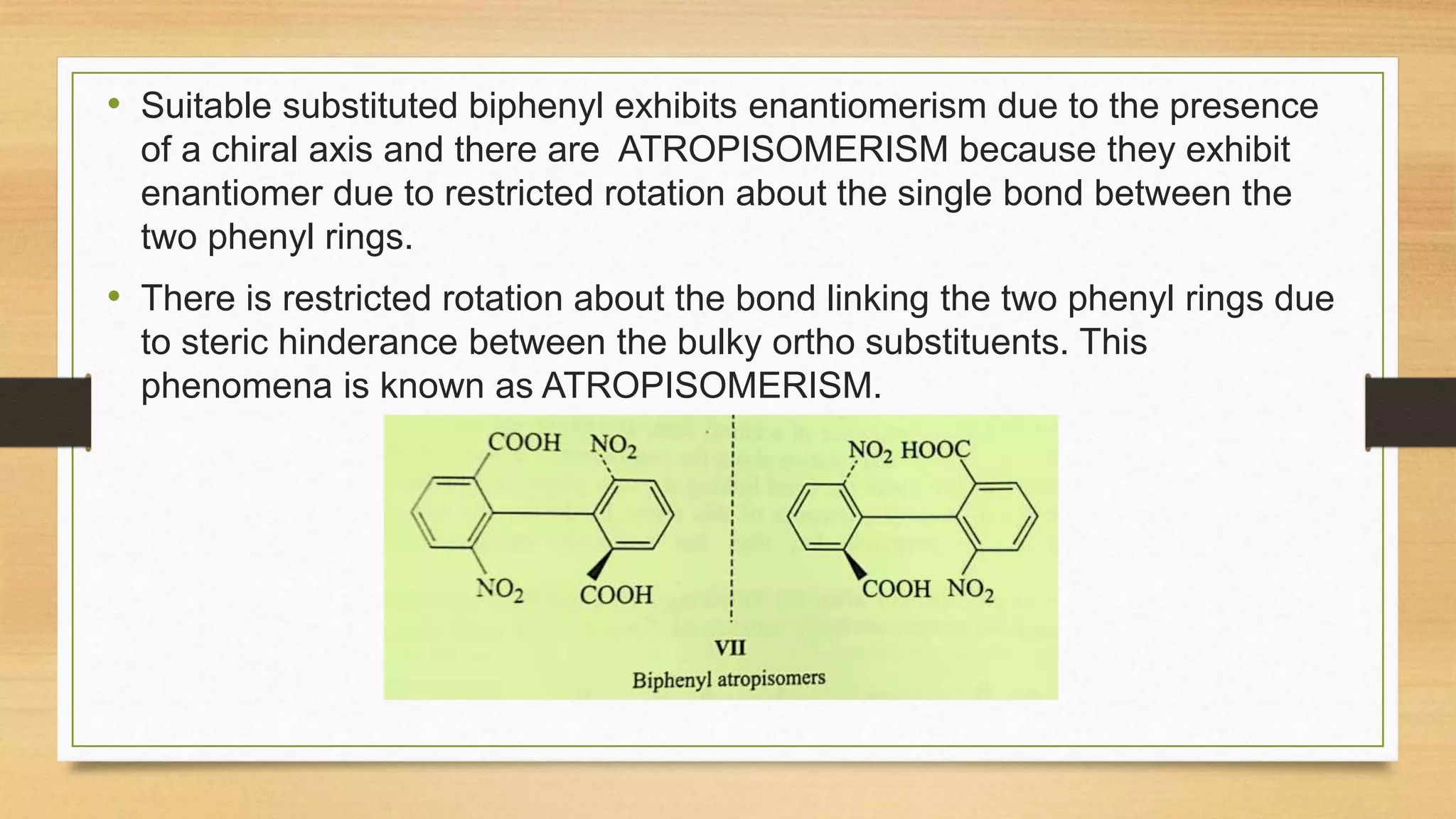
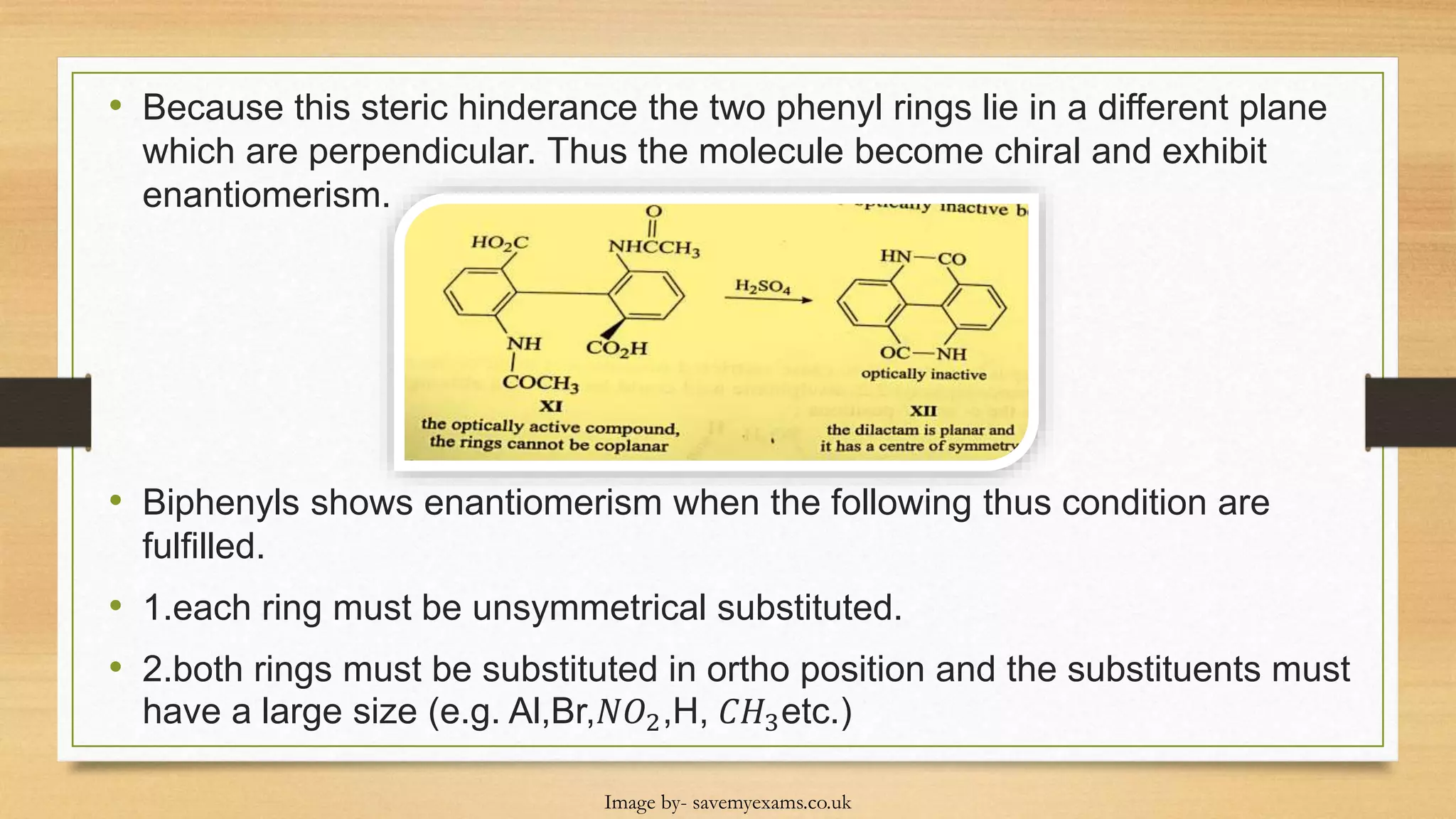
This document discusses the stereochemistry of allenes, spiranes, and biphenyls. It explains that allenes with different substituents on the terminal carbons can exhibit chirality and enantiomers. Spiranes can also show chirality and optical isomerism if they have different substituents. Biphenyls become chiral when large substituents in the ortho position prevent free rotation of the phenyl rings, leading to atropisomerism with a chiral axis and restricted rotation.