This document discusses substitution reactions in square planar complexes, primarily focusing on platinum(II) complexes. It describes the associative mechanism of these reactions, detailing types like oxidative addition, electrophilic substitution, and nucleophilic substitution, as well as the involvement of stable intermediates. Additionally, the document outlines the rate laws for these reactions and differentiates between solvent path and direct displacement mechanisms.

![Substitution reaction occurs via associative mechanism (SN2).
Three types:
1. Oxidative addition followed by reductive elimination
[PtII (PR3)2 (R)Cl] + Cl2 → [PtIV (PR3)2 (R)Cl3] → [PtII (PR3)2Cl2]
+ RCl
2. Electrophilic substitution
[PtII (PR3)2 (R)Cl] + HgCl2 → [PtII (PR3)2 Cl2] + RHgCl
3. Nucleophilic substitution
[PtII (PR3)2 (R)Cl] + NH3 → [PtII (PR3)2 (R)NH3]+ + Cl-](https://image.slidesharecdn.com/15part1substitutionreactionsinsquareplanarcomplexes-230424031015-921a09f2/85/Part-1-Substitution-reactions-in-square-planar-complexes-pptx-3-320.jpg)
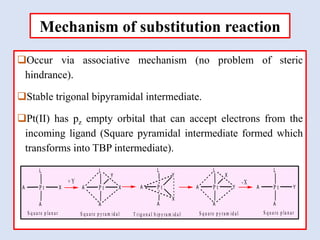
![Mechanism of substitution reaction
Stereochemistry
Pt
A X
A
L
Pt
A Y
A
L
Pt
L X
A
Pt
L Y
A
A
A
Pt
A
L
Y
X
A
Pt
A
A
Y
X
L
+Y
+Y
-X
-X
cis-[Pt(A)2XL] cis-[Pt(A)2YL]
trans-[Pt(A)2YL]
trans-[Pt(A)2YL]](https://image.slidesharecdn.com/15part1substitutionreactionsinsquareplanarcomplexes-230424031015-921a09f2/85/Part-1-Substitution-reactions-in-square-planar-complexes-pptx-5-320.jpg)

![[Pt(A)2LX] + H2O → [Pt(A)2L(H2O)]+ + X-
[Pt(A)2L(H2O)]+ + Y- → [Pt(A)2L(Y)] + H2O
Rate of reaction (r1) = k1 [Pt(A)2LX] [H2O]
k1 [Pt(A)2LX]
Mechanism of substitution reaction
Slow
Fast
Solvent path](https://image.slidesharecdn.com/15part1substitutionreactionsinsquareplanarcomplexes-230424031015-921a09f2/85/Part-1-Substitution-reactions-in-square-planar-complexes-pptx-7-320.jpg)
![Mechanism of substitution reaction
Fast
Direct path
[Pt(A)2LX] + Y- → [Pt(A)2LXY]-
[Pt(A)2LXY]- → [Pt(A)2L(Y)] + X-
Rate of reaction (r2) = k2 [Pt(A)2LX] [Y-]
Slow
k2
Fast](https://image.slidesharecdn.com/15part1substitutionreactionsinsquareplanarcomplexes-230424031015-921a09f2/85/Part-1-Substitution-reactions-in-square-planar-complexes-pptx-8-320.jpg)
![Rate of substitution reaction = r1 + r2
= k1 [Pt(A)2LX] + k2 [Pt(A)2LX] [Y-]
= [Pt(A)2LX] (k1 + k2 [Y-])
Two term rate law
1. k1 = first order rate constant (solvent path)
2. K2 = second order rate constant (direct path)
The reaction is pseudo first order (concentration of Y- is constant)
Mechanism of substitution reaction](https://image.slidesharecdn.com/15part1substitutionreactionsinsquareplanarcomplexes-230424031015-921a09f2/85/Part-1-Substitution-reactions-in-square-planar-complexes-pptx-9-320.jpg)

