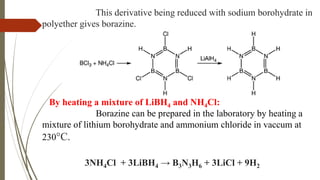
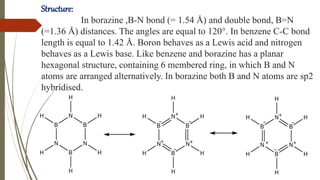
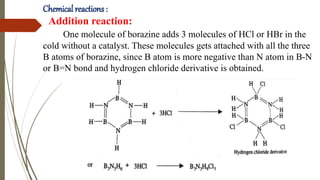
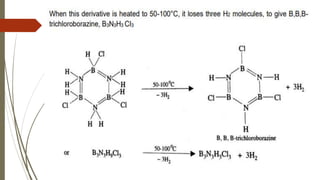
Borazine, also known as borazole, is an inorganic compound with the formula B3H6N3, analogous to benzene. It can be synthesized through various methods involving diborane and ammonia and has a volatile liquid form with distinct physical properties. Borazine has applications in ceramics and as a precursor for hexagonal boron nitride thin films, and can undergo various chemical reactions, including hydrolysis and polymerization.


![Preparation:
Stock and Pohlands method:
By the action of NH3 on diborane in 1:2 ratio. The
adduct B2H6.NH3 is first formed, which then gets decomposed by
heating in a closed tube at 200°C.
3 B2H6 + 6 NH3 → 3[B2H6.NH3] → 2 B3H6N3 + 12 H2
Heating BCl3 with NH4Cl:
Heating boron trichloride with ammonium chloride in
chlorobenzene in the presence of Fe, Ni, or Co catalyst at 140°C
trichloroborazine is formed.](https://image.slidesharecdn.com/borazine-201222124055/85/Borazine-3-320.jpg)

![Formation of adduct:
Borazine forms an adduct with methanol. This adduct undergoes pyrolysis
with the elimination of hydrogen and gives B-trimethoxy-borazine.
Polymerization:
Borazine heated at 70 °C expels hydrogen gas
with formation of polyborazylene.
n B3N3H6 → [B3N3H4]n](https://image.slidesharecdn.com/borazine-201222124055/85/Borazine-13-320.jpg)

![NET and GATE exam Questions:
1) BCl3 with NH4Cl gives product A which upon reduction by NaBH4 gives product
B. Product B upon reacting with HCl affords compound C, which is
a) Cl3B3N3H9
b) (ClBNH)3
c) (HBNH)3
d) (ClH)3B3N3CClH3
2) Heating mixture of NH4Cl gives one liquid product (X), along with other
products. Compound (X) is
a) NH4[BH4]
b) [(NH3)2BH2][BH4]
c) N3B3H6
d) N3B3H12](https://image.slidesharecdn.com/borazine-201222124055/85/Borazine-15-320.jpg)
![3) BCl3 and NH4Cl were reacted at 140oC to give compound X, which then
treated NaBH4 gave compound Y, X and Y ?
a) X= B3N3H6Cl3 , Y= B3N3H6
b) X= B3N3H9Cl3 , Y= B3N3H6
c) X= B3N3H9Cl3 , Y= B3N3H12
d) X= B3N3Cl6 , Y= B6N3H6
4) 3NH4Cl + BCl3 X Y ; X,Y ?
a) [HB(NH)]3, [H(OH)B(NH2)]3
b) [HB(NH)]3, [HB(NH2OH)]3
c) (NH4)(H)2(BH2)3,[H(OH)(NH2OH)]3
d) (NH4)(H)2(BH2)3, [HB(NH2OH)]3
C6H5Cl
LiBH4
3H2O](https://image.slidesharecdn.com/borazine-201222124055/85/Borazine-16-320.jpg)
