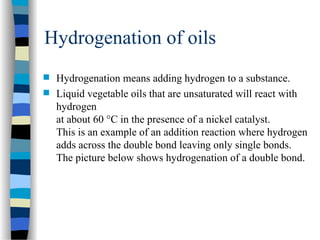
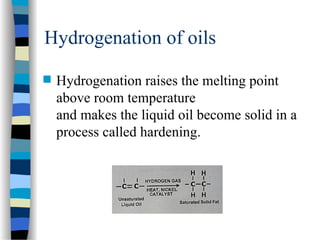
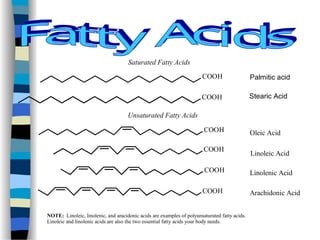
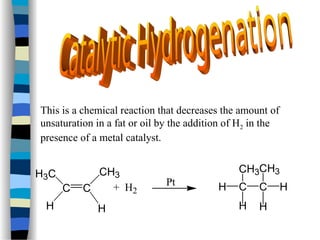
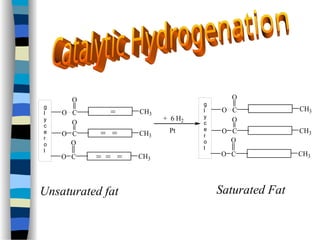
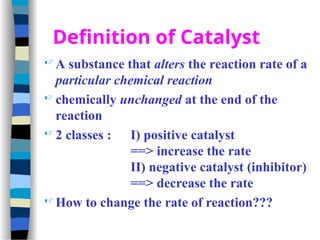
This document outlines the definition and characteristics of catalysts, which are substances that alter the reaction rates of chemical reactions without changing themselves. It covers different types of catalysis, including positive, negative, homogeneous, heterogeneous, and enzyme catalysis, as well as their industrial applications like the Haber and Ostwald processes. Additionally, it explains the mechanisms involved in enzyme catalysis, including the Michaelis-Menten equation and various factors affecting reaction rates.

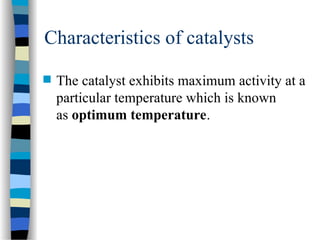
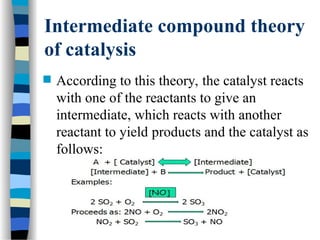

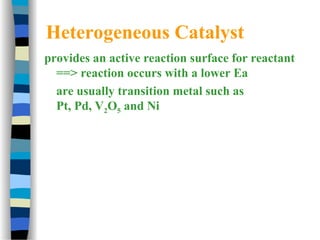
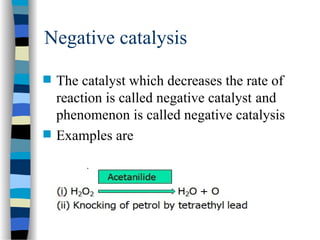
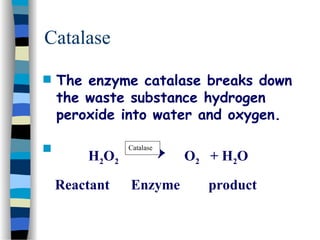

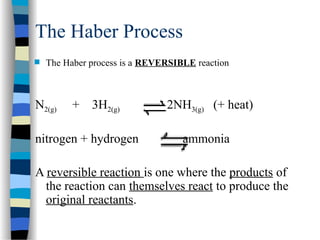
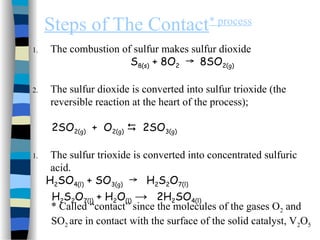
![The Michaelis-Menten Equation
[E] + [S] [ES] [P] + [E]
k2
k-1
k1
Assumption: k-1 >> k2 i.e. the equilibrium of [E], [S] and [ES] is not
affected by k2:
KS = =
k-1
k1
[E] [S]
[ES]
KS = dissociation constant
[ES] = „Michaelis-Menten“ complex
Since we assume equilibrium it follows:
[E] [S] k1 = [ES] k-1 solving for [E] =
k-1
k1
[ES]
[S]
(1)
In addition we know that: [E]total = [E] + [ES] (2)
This relationship is called the „enzyme conservation equation“](https://image.slidesharecdn.com/catyalisis-241106181150-2990c34a/85/catyalisis-presentation-in-engineering-ppt-28-320.jpg)
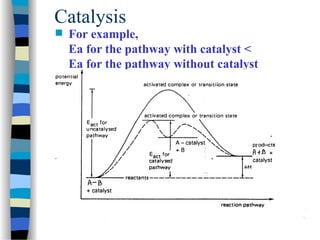
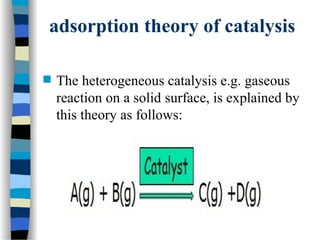
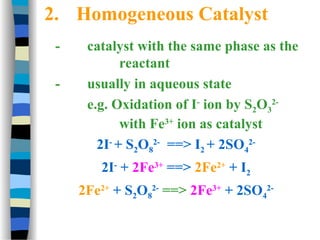
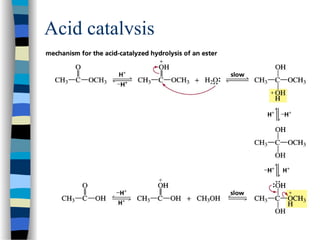
![The Michaelis-Menten approach
[E] =
k-1
k1
[ES]
[S]
(1)
[E]total = [E] + [ES] (2)
Solving equation (2) for [E] and substituting [E] in equation (1):
[E]total = [ES] (1 + )
k-1
k1 [S]
We also know that the velocity of the reaction equals:
v = k2 [ES]
(3)
(4)
Solving equation (3) and (4) for [ES] and then substituting [ES] in
equation (3) with [ES] = v / k2 then yields:](https://image.slidesharecdn.com/catyalisis-241106181150-2990c34a/85/catyalisis-presentation-in-engineering-ppt-29-320.jpg)
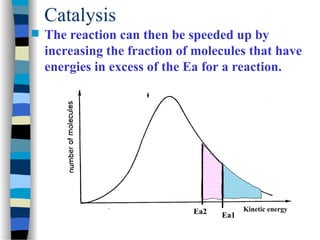
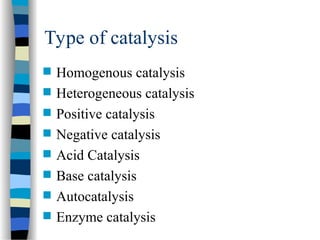
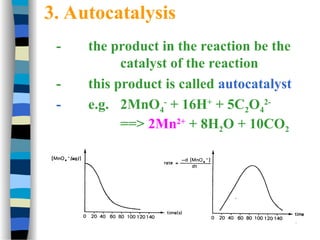
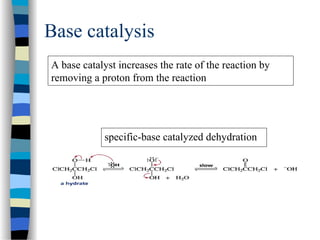
![k2
(1 + )
k-1
k1 [S]
v = =
[E]total k2 [E]total
[S]
k-1
k1
[S]
We define k-1/ k1 as KM, the Michaelis-Menten constant and the
maximal velocity as vmax = k2 [E]total
This simplifies the above equation to:
v =
k2
vmax [S]
[S] + KM
if [S] >> KM then v = vmax
if [S] = KM then v =
vmax
2
Therefore KM can be viewed as the substrate concentration with half-
maximal velocity (dimension M, typically mM to nM)
+](https://image.slidesharecdn.com/catyalisis-241106181150-2990c34a/85/catyalisis-presentation-in-engineering-ppt-30-320.jpg)
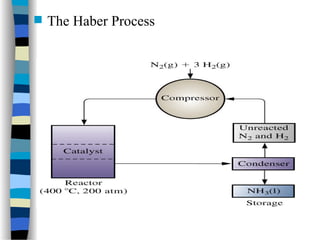
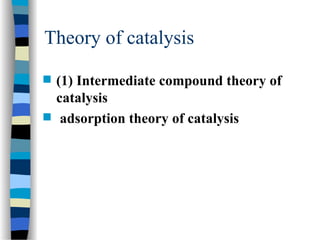
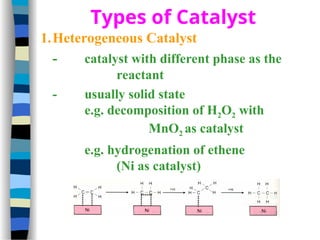
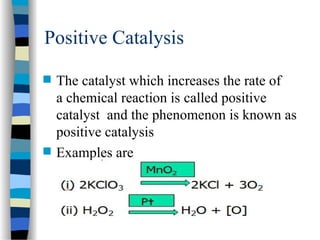
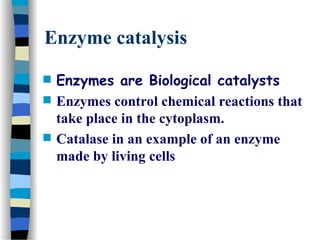
![Michaelis-Menten plot
v
[S]
vmax
KM
vmax
2
1st order zero order
Linear plot of substrate concentration versus velocity
yields a hyperbolic relationship:](https://image.slidesharecdn.com/catyalisis-241106181150-2990c34a/85/catyalisis-presentation-in-engineering-ppt-31-320.jpg)