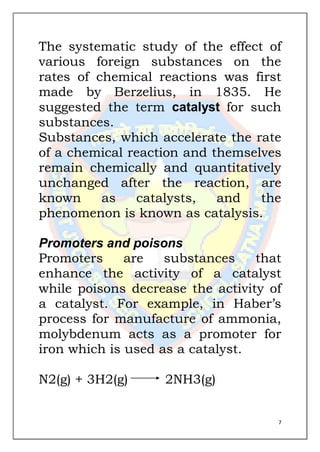
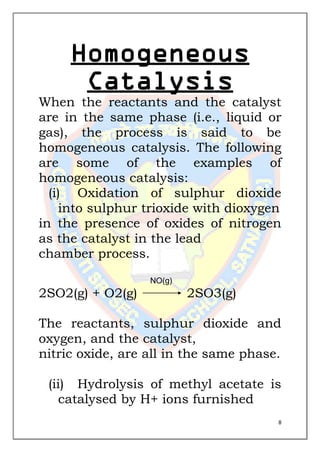
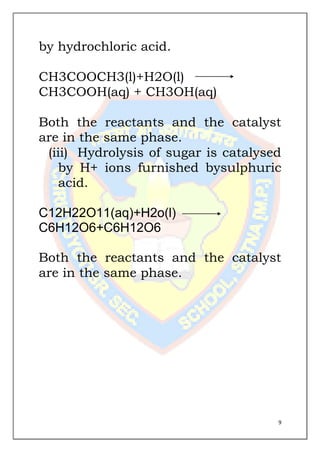
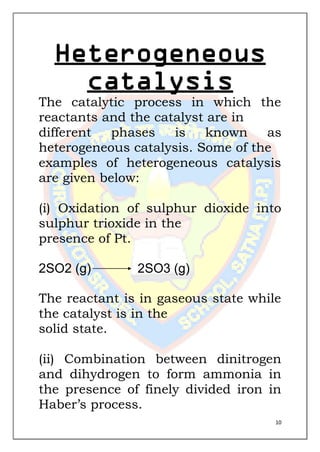
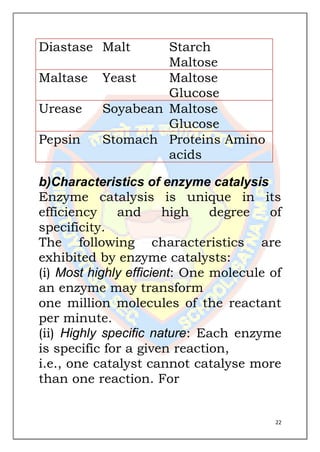
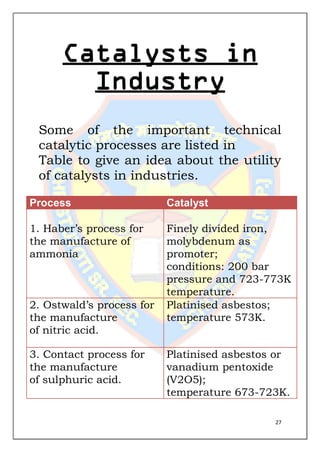
This document is a chemistry project submitted by Shivam Yadav of class 12 on the topic of catalysis. It includes an acknowledgment, certificate, contents listing, and several sections discussing different types of catalysis like homogeneous catalysis, heterogeneous catalysis, and enzyme catalysis. It also covers concepts like the adsorption theory of heterogeneous catalysis, shape selective catalysis using zeolites, and examples of catalysts used in important industrial processes.