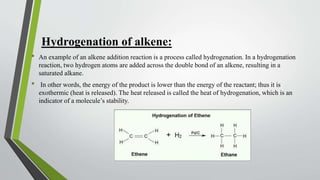
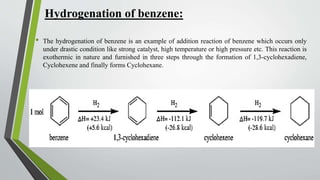
The document discusses the process of hydrogenation, a chemical reaction involving the addition of hydrogen to organic compounds, typically facilitated by catalysts like nickel, palladium, or platinum. It details the hydrogenation of alkenes, alkynes, and benzene, explaining mechanisms and conditions under which these reactions occur, while emphasizing the exothermic nature of the process and the importance of stopping reactions at specific stages to prevent over-hydrogenation. Overall, the document outlines the fundamentals and applications of hydrogenation in organic chemistry.