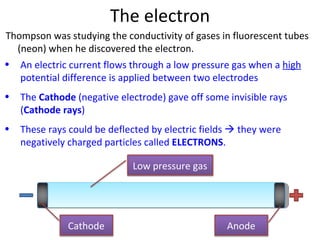
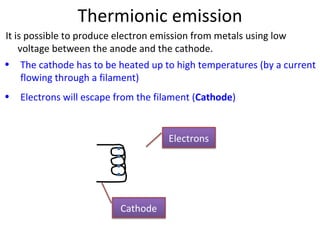
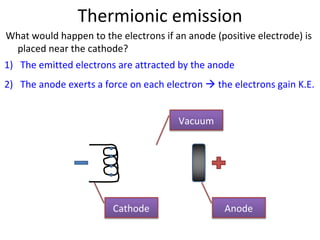
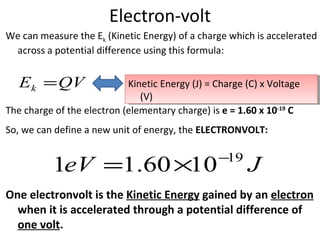
1. The document discusses the discovery of electrons and their behavior when accelerated by electric and magnetic fields, defining the electronvolt unit of energy.
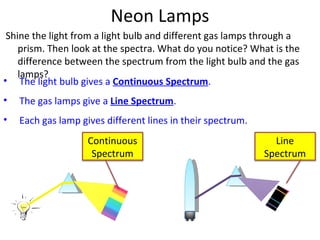
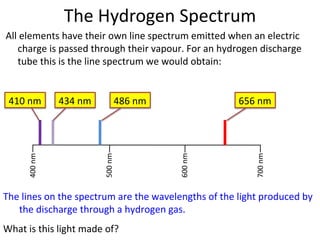
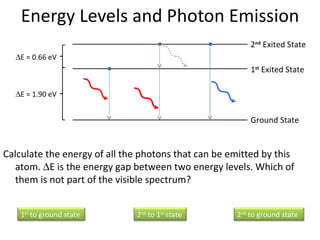
2. Different gases emit different colored light due to their unique line emission spectra, which results from electrons dropping between distinct energy levels in atoms.
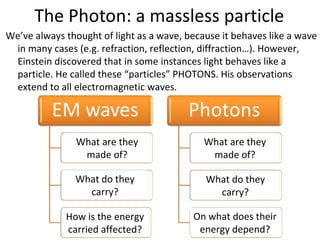
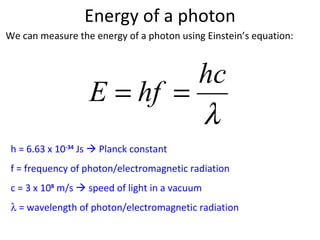
3. Photons are massless particles that carry electromagnetic energy in quantized amounts depending on their frequency or wavelength.