Embed presentation
Downloaded 31 times
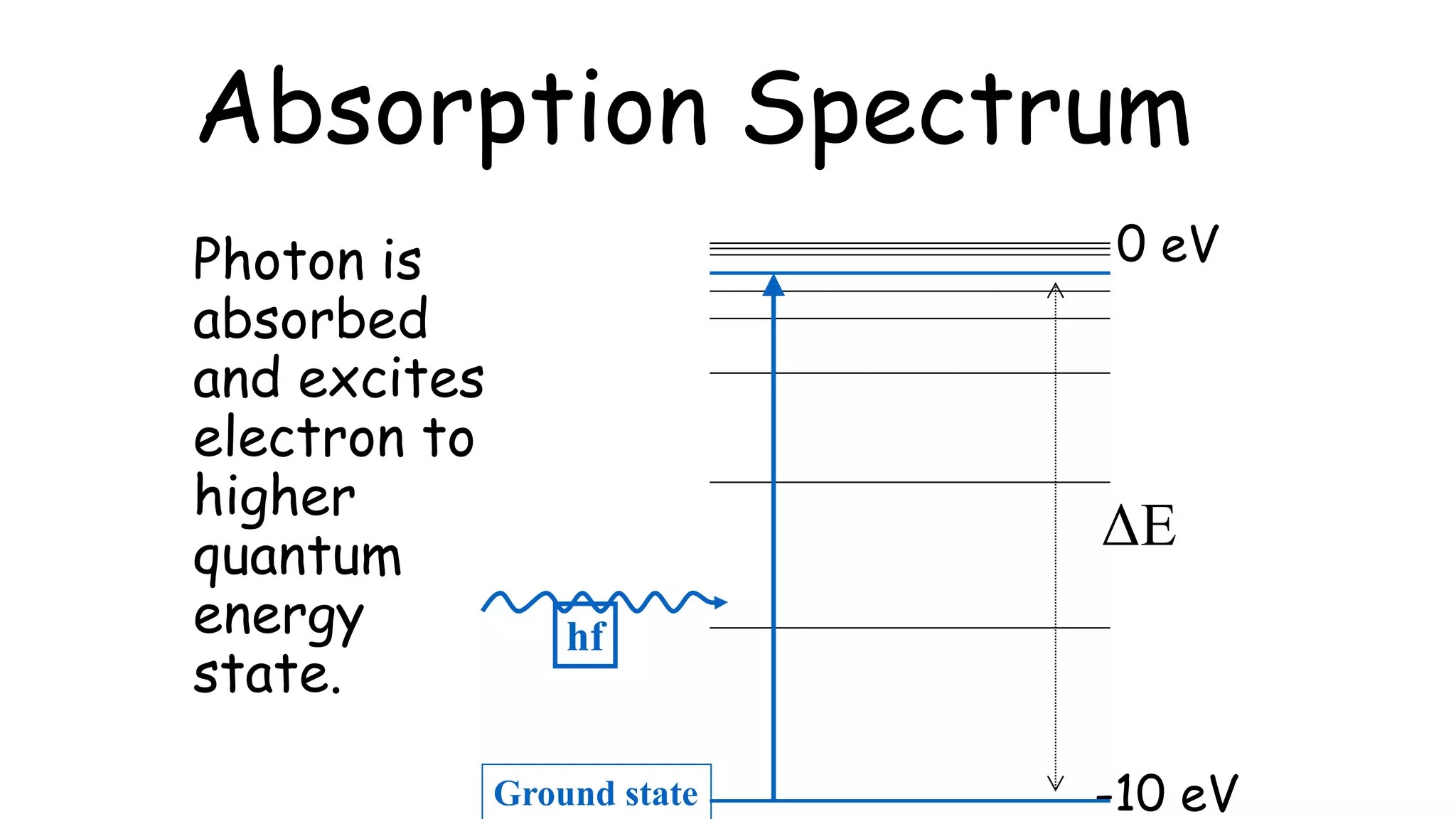

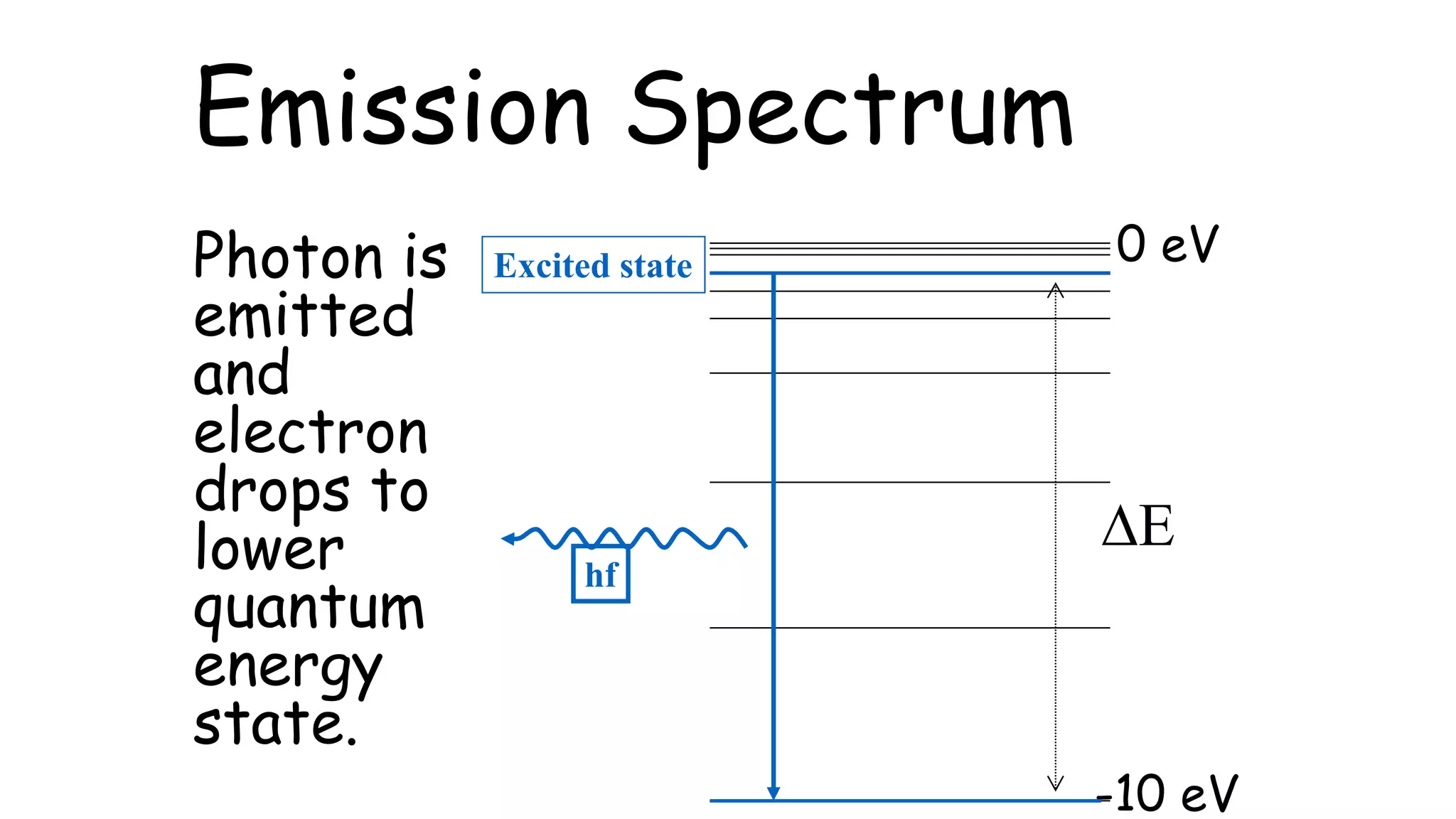


An absorption spectrum occurs when a photon is absorbed, exciting an electron to a higher quantum energy state. Absorption spectra involve atoms increasing in energy level. An emission spectrum occurs when a photon is emitted as an electron drops to a lower quantum energy state, with emission spectra involving electrons decreasing their energy level. The document also provides a conversion between electronvolts (eV) and joules (J).




