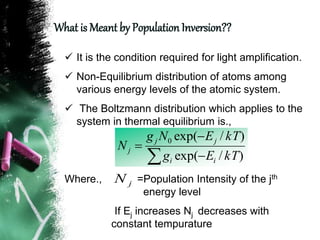
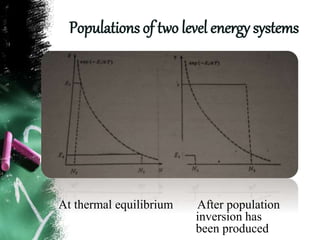
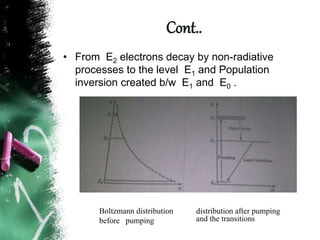
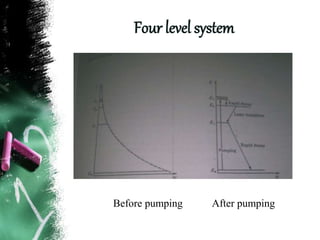
Population inversion is a non-equilibrium distribution of atoms among energy levels that is required for light amplification. It occurs when more atoms are in an excited, higher energy state than in a lower energy ground state. Various pumping techniques can induce population inversion by exciting atoms to higher energy levels. This includes laser excitation, optical pumping, electrical discharges, and chemical reactions. Once inverted, stimulated emission can occur, enabling applications like lasers that rely on stimulated amplification of light.