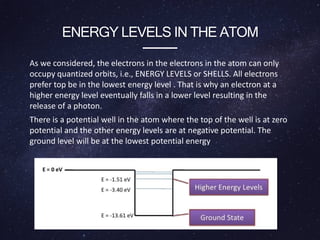
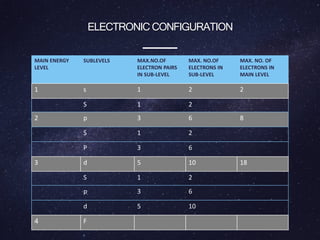
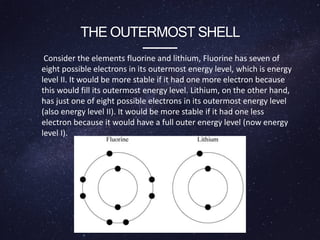
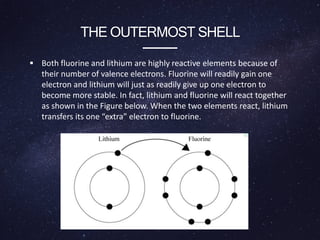
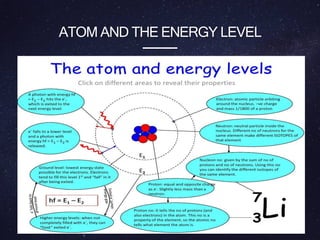
Energy levels (or electron shells) are specific distances from an atom's nucleus where electrons may exist, akin to steps on a staircase. Electrons prefer the lowest energy level, and those in higher levels will release energy, often as photons, when they fall to lower levels. Valence electrons in the outermost energy level are crucial for chemical reactivity and stability, exemplified by the behavior of fluorine and lithium.